Distinct mechanisms determine organ left-right asymmetry patterning in an uncoupled way
Abstract
Disruption of Nodal in the lateral plate mesoderm (LPM) usually leads to left-right (LR) patterning defects in multiple organs. However, whether the LR patterning of organs is always regulated in a coupled way has largely not yet been elucidated. In addition, whether other crucial regulators exist in the LPM that coordinate with Nodal in regulating organ LR patterning is also undetermined. In this paper, after briefly summarizing the common process of LR patterning, the most puzzling question regarding the initiation of asymmetry is considered and the divergent mechanisms underlying the uncoupled LR patterning in different organs are discussed. On the basis of cases in which different organ LR patterning is determined in an uncoupled way via an independent mechanism or at a different time, we propose that there are other critical factors in the LPM that coordinate with Nodal to regulate heart LR asymmetry patterning during early LR patterning.
Also watch the Video Abstract.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- AP
-
- anterior-posterior
-
- DV
-
- dorsal-ventral
-
- GRP
-
- gastrocoel roof plate
-
- Has2
-
- hyaluronan synthase2
-
- KV
-
- Kupffer's vesicle
-
- LPM
-
- lateral plate mesoderm
-
- LR
-
- left-right
-
- PD
-
- Parkinson's disease
-
- RA
-
- retinoic acid
-
- So
-
- somite stage
Introduction
Although the vertebrate body appears to be bilaterally symmetrical, left-right (LR) asymmetry manifests in both the structure and placement of the internal organs 1, 2 as well as in the nerves of the brain 3, 4. Disruption of LR asymmetry leads to disorders of organ laterality, including situs inversus and heterotaxy 5-7, and it can even result in, or be accompanied by, serious birth defects such as congenital cardiac disease 8, dyslexia, and schizophrenia 9-11. Recently, alteration of the LR asymmetric structure in the brain was reported to be a high risk factor for Alzheimer's disease (AD) in the elderly 12, 13 and to be associated with infantile autism 14, 15. Furthermore, an altered pattern of asymmetric dopaminergic neurons has been found in patients with Parkinson's disease (PD) 16. Thus, in order to fully understand the pathogenesis of LR-related diseases such as PD and AD, it is necessary to elucidate the complicated process of LR patterning and the underlying mechanisms involved.
During the past several decades, numerous theoretical models have been proposed to explain the establishment of LR asymmetric patterning in different vertebrates and invertebrates 7, 17-20. A number of intercellular signaling pathways, such as Nodal, Notch, FGF, Wnt, BMP, and Hedgehog, have been reported to be involved in the regulation of LR asymmetric patterning and manipulation of these signals has been demonstrated to result in asymmetric gene expression defects and sequential organ LR defects 2, 17, 21-26. Although several early studies proposed theoretical models to describe the processes involved in the initiation, transmission, and maintenance of asymmetric information in the mouse, zebrafish, and Xenopus, recent research has led to even more questions regarding LR patterning 18, 19. In this review, the process of LR asymmetry patterning is briefly summarized and the most puzzling issue regarding the initiation of the asymmetric cascade is discussed. This is followed by a detailed analysis of the different mechanisms involved in the uncoupled regulation of individual organ LR patterning (“uncoupled” signifies that different organ LR patterning defects do not always occur simultaneously and that the degree to which organ LR patterning defects occur is not the same when different types of signaling are disturbed). Finally, it is hypothesized that other critical unilateral signals may exist in the lateral plate mesoderm (LPM) that coordinate with the left-sided Nodal and regulate LR patterning in the heart.
The establishment of organ LR asymmetry
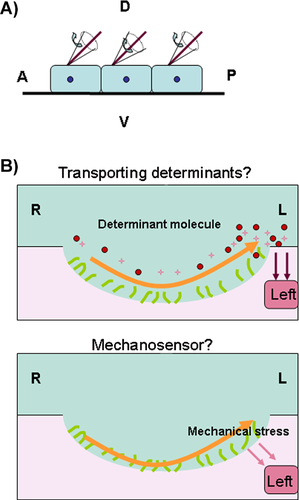
In general, several critical steps must be properly coordinated for organ LR asymmetry patterning in vertebrates 17, 19, 27, 28 (Fig. 1). These include the existence of molecular gradients in the anterior-posterior (AP) and dorsal-ventral (DV) axes 18, 19, 29, the initiation of the asymmetric information in the node (gastrocoel roof plate (GRP) in Xenopus or Kupffer's Vesicle (KV) in zebrafish) 2, 30-32 (Fig. 2), the transmission and maintenance of asymmetric gene expression in the LPM 2, 7, 33 and organ primordia asymmetric migration 19, 33. Brown and Wolpert proposed that the first step of this process involves a chiral “F-molecule”, which exists in cells that transport intracellular constituents. This creates a molecular gradient in whole cells and tissue, allowing AP and DV axes to form 21, 29. Because cilia tilt in a posterior way at an average angle of 30° in the node 34, 35 and appear to be translated by the positional cues of preexisting AP and DV axes 36 (Fig. 2A), it is hypothesized that the molecular gradients of the these axes determine ciliogenesis 36. The disruption of a number of signaling molecules results in defective cilia formation and in the sequential abnormal expression pattern of nodal and the nodal downstream genes pitx2, lefty2, and lefty1 et al., resulting in the different LR laterality defects in organs 21, 23, 24, 37-40. Hence, correct ciliogenesis (or cilia function), and the mild expression of Nodal in or near the node, result in the asymmetric expression of nodal as well as its downstream genes pitx2, lefty2, and lefty1 et al. in the LPM and head 17, 33. The asymmetric downstream genes function as directors, guiding the asymmetric migration of the organ primordia. In addition to proper maintenance of unilateral asymmetric expression in nodal and its downstream genes, an intact midline (including the notochord and floorplate) is also required 7, 17. Disruption of the midline structure or midline gene expression disturbs the left lateral gene expression pattern and the sequential organ LR patterning 41-44. Although the whole process is broadly conserved in most vertebrates, the underlying mechanism varies greatly between species. For example, in chickens and pigs there is no node-like tissue or functional cilia to initiate the asymmetric cascade 45, 46. Therefore, much about the complicated process of LR patterning in the animal world remains unknown.
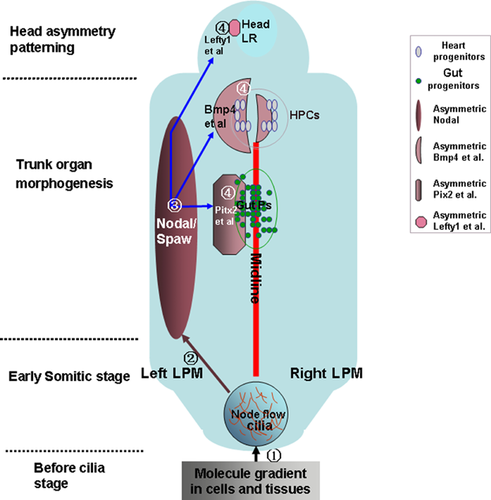
 ). In this stage, mild Nodal asymmetry appears near the node, which is expanded (step
). In this stage, mild Nodal asymmetry appears near the node, which is expanded (step  ) and amplified (step
) and amplified (step  ) in the left LPM, but inhibited in the right LPM. The asymmetric Nodal is maintained by the intact midline (red line). Finally, in the organ morphogenesis stage, including early trunk and later neural tissue morphogenesis, the left-sided Nodal influences multiple downstream genes (pitx2, lefty2, lefty1, bmp4, etc.) to separately regulate specific organ LR patterning (step
) in the left LPM, but inhibited in the right LPM. The asymmetric Nodal is maintained by the intact midline (red line). Finally, in the organ morphogenesis stage, including early trunk and later neural tissue morphogenesis, the left-sided Nodal influences multiple downstream genes (pitx2, lefty2, lefty1, bmp4, etc.) to separately regulate specific organ LR patterning (step  ).
).
Asymmetry is initiated in multiple ways
Several assumptions were made when explaining the initiation of the LR asymmetric cascade and the subsequent LR asymmetry patterning in different animal models 1, 2, 20. It has been hypothesized that the unidirectional movement of the cilia causes chiral fluid flow in the node in the mouse (the KV in the zebrafish and the GRP in Xenopus) and that it is this counter-clockwise fluid flow that initiates the asymmetric expression of nodal (Fig. 2), as well as the downstream genes in the LPM and head, which subsequently directs the organ primordia to migrate 47-52. However, this theory has been recently challenged 18-20. First, node-related block tissue and cilia do not exist in all vertebrates. In the chicken, asymmetric cell movement around Hensen's node 46 and the physiological asymmetries (such as H+ flux) at earlier stages 53, 54, result in asymmetric gene expression 46. Second, even in vertebrates with “node” tissue, such as mice, when the nodal flow is disturbed, the rate of heart situs defects is higher than the rate of all organ situs defects in Xenopus 19, 31, indicating that asymmetry in Xenopus might be initiated in some other way than via the only node/cilia. Additional evidence shows that this assumption can also be made for mice and zebrafish. In the mouse Nek8 mutant, the left-sided marker genes nodal and pitx2 were disturbed, but the node and cilia were intact 55. In zebrafish pegasus morphants, KV formation and ciliogenesis were not reported to be defective, though the left-sided marker genes and organ laterality were randomized 56. Third, in contrast to a few studies imparting a direct role to nodal flow in LR patterning 31, 57, 58, many gaps in the cilia literature have led to questions about an exclusive causative link between the cilia and LR asymmetry patterning 18, 27. Even artificial nodal flow has been found to result in LR asymmetry defects in mice 57, while it is possible that, besides a defect in nodal flow, other unknown events occur in the node that may be critical for LR asymmetric patterning in the mouse 59, 60. Evidence from zebrafish also suggests that there is not an exclusive, causative link between the cilia and LR asymmetric patterning. Although the experiments targeting the molecular reagents in the KV/cilia in zebrafish resulted in an LR patterning defect 41, 61-67, none of these studies directly proved that defective ciliogenesis and the resulting perturbed nodal flow were the only reason for the observed LR asymmetric defects. Fourth, even in “node” tissue animal models, the LR asymmetric information has been shown to exist far earlier than the node stage. In Xenopus embryos, the earlier LR asymmetry exists in the localization of H+/K+-dependent ATPase, the K+ channel components KCNQ1 and KCNE1, and in H+-V-ATPase at the early cleavage stage 54, 68, 69. Earlier LR asymmetry also exists in Xenopus embryos during the distribution of the neurotransmitter serotonin at the 32-cell stage before cilia formation. All of these molecules have been found to be involved in regulating LR asymmetry patterning in frog embryos 70 and serotonin has been reported to have cilia-independent roles in Xenopus LR asymmetry patterning 71. H+/K+ ATPase has also been reported to have a role in regulating LR asymmetry patterning in zebrafish, where H+/K+ ATPase acts before the start of zygotic transcription and seems to work in parallel with the cilia, during the early somitic stage, to regulate LR asymmetry patterning 72. In addition, a mutation (atp1a1am883, the Na, K-ATPase subunit atp1a1a) in zebrafish 73 has been shown to cause isomerism of internal organs and bilateral expression of left-sided spaw, with normal monocilia movement and fluid circulation in the KV. This further implies that other critical signals exist, besides “node flow”, in the early development of “node” tissue animals.
As mentioned above, symmetry breakage can occur before or during the cilia stage, however, there may be a special mechanism for the neural asymmetry patterning that occurs later in the neurogenesis stage. Recently, asymmetric migration of the parapineal nucleus in zebrafish fgf8 mutants (acerebellarti282) was reported to be disturbed even when left-sided nodal activity in the LPM was normal 74. Further evidence in fgf8 mutants indicated that the mild asymmetric expression of fgf8, and of its receptor fgfr4 in the epithalamus, was capable of directing the asymmetric migration of the parapineal nucleus 74. Thus, besides the early asymmetric information, the later asymmetric information such as fgf8, which works as the initiator but not the mediator of early asymmetry regulators, is involved in LR patterning of neural organs.
In conclusion, all these studies indicate that asymmetric information might appear both far earlier and far later than the cilia stage. Different asymmetric instructions for the establishment of individual organ LR asymmetry appear at distinct time points, especially in the case of specific asymmetric genes in the head 74, implying that different organ laterality may be determined in an uncoupled way.
Asymmetric morphogenesis of organs may be independent of left-sided Nodal
Asymmetric morphogenetic defects of the heart are caused not only by disruption of Nodal in the LPM
It is well known that disruption of Nodal in the LPM causes organ LR patterning defects. However, even when Nodal is normal in the LPM, defects in heart laterality still occur in some cases. Although Nodal signaling in the LPM is important for regulating the asymmetric migration of cardiac primordia, the cellular characteristics and behavior of the heart primordia are also critical for heart LR patterning, and these are regulated by factors independent of Nodal. For example, in zebrafish natter mutants that lack functional Fibronectin, the myocardial primordium loses coherence and fails to migrate to the midline, leading to cardia bifida 75, 76. Hyaluronan synthase2 (has2), expressed in the myocardial cells located at the leading edge of the cardiac cone, is also involved in regulating myocardial cell determination and rotation, but is not involved in regulating left-sided nodal or lefty2 and pitx2 77. In zebrafish, the mutations heart and soul (has) and nagie oko (nok) cause the loss of apicobasal polarity in myocardial progenitors, leading to the nondirectional migration of myocardial cells (most myocardial cells are immobile and remain at the embryonic midline, and the peripheral myocardial cells lose contact with neighboring cells and initiate nondirectional single-cell migration). Ultimately, this leads to an LR patterning defect in the heart 78. However, the asymmetric Nodal defect has not been reported in the LPM, and thus a left-sided Nodal defect is not the only reason for the heart laterality defect. Disturbing the coherent apicobasal polarity of the cardiac primordium can also lead to an LR patterning defect in the heart.
Wnt and BMP signals play crucial roles in regulating Node/cilia function prior to early somitogenesis 23, 24, 79, 80 and in determining the fate of heart progenitor cells and heart morphogenesis 81-84. Loss of either of these two signals results in an LR-asymmetric defect of the heart, accompanied by disturbed Nodal signaling in the LPM and improper cardiac primordium formation. Therefore, it is difficult to discern whether the defective cardiac LR asymmetry is the result of a combination of a disturbed Nodal cascade and incorrect cardiac primordium formation caused by the disruption of Wnt or BMP signaling. Recently, we found that blocking retinoic acid (RA) signaling before the 2-somite stage (2So) in zebrafish caused an extremely linearized heart phenotype with Nodal activity on both sides of the LPM and an increased heart progenitor 37. However, the asymmetric expression pattern of the more direct factor, bmp4, was not altered in the heart field. Thus, we inferred that the linearized heart phenotype was possibly due to defective cell fate determination or some other cardiac primordium character defect(s) 37, 85-87, while not due to Nodal/Spaw-Bmp4 cascade defect (Fig. 3). The idea that heart asymmetry patterning may be related to cardiac primordium determination or another reason, such as cell coherence and apicobasal polarity, is also consistent with results from earlier research done in the mouse, where Biben et al. reported that the intrinsic heart LR patterning regulator, eHand, is regulated by Nkx2.5 88. Thus, it is possible that cardiac progenitor determination and some other unknown factor, both of which are independent of Nodal signaling in the LPM, are involved in heart LR asymmetric patterning.
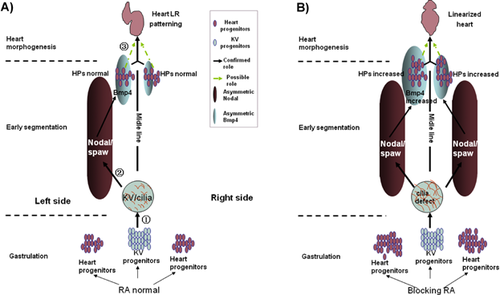
 ), which controls the node flow in KV and the subsequent expression of left-sided Nodal in the LPM at the early segmentation stage (step
), which controls the node flow in KV and the subsequent expression of left-sided Nodal in the LPM at the early segmentation stage (step  ). Before 2So, RA also regulates the commitment of the heart progenitors; this function may be combined with the asymmetric Nodal and the downstream gene bmp4 in the LPM at the early So, and together they regulate the asymmetric migration of heart progenitors (in step
). Before 2So, RA also regulates the commitment of the heart progenitors; this function may be combined with the asymmetric Nodal and the downstream gene bmp4 in the LPM at the early So, and together they regulate the asymmetric migration of heart progenitors (in step  dark arrow and yellow-green dotted arrow). B: When blocking RA activity before 2So, more cells were committed to be heart progenitors (step
dark arrow and yellow-green dotted arrow). B: When blocking RA activity before 2So, more cells were committed to be heart progenitors (step  ). In the cilia stage, the cilia length increased and node flow was disturbed, which subsequently resulted in bilateral Nodal/Spaw in the LPM; however, the asymmetric expression of the downstream factor, bmp4, remained normal, though the reason for this is not known (even blocking RA increased the expression of bmp4). Thus, the increase in heart progenitors, along with asymmetric bmp4 expression, might have resulted in the linearized heart phenotype observed in embryos treated with BMS453 before 2So (step
). In the cilia stage, the cilia length increased and node flow was disturbed, which subsequently resulted in bilateral Nodal/Spaw in the LPM; however, the asymmetric expression of the downstream factor, bmp4, remained normal, though the reason for this is not known (even blocking RA increased the expression of bmp4). Thus, the increase in heart progenitors, along with asymmetric bmp4 expression, might have resulted in the linearized heart phenotype observed in embryos treated with BMS453 before 2So (step  , yellow-green dotted arrow). So: somite stage.
, yellow-green dotted arrow). So: somite stage.Gut asymmetry defects can occur when Nodal is normal in the LPM
Similar to heart morphogenesis, asymmetric looping in the gut is regulated not only by the asymmetric Nodal cascade, but also by other factors that directly influence the character of gut progenitors and/or the surrounding cells, affecting such things as the extracellular matrix and cell-cell adhesion, both of which are important for correct migration of the gut primordium to occur 89. In the chick embryo, left-sided Nodal instructs the epithelial cells of the left dorsal mesentery to adopt high columnar shapes and the mesenchymal cells to condense during the midgut looping stage, whereas the right-sided epithelial cells are cuboidal 90. These asymmetric cell characteristics directly lead to a trapezoidal shape being formed by the mesentery, which tilts the primitive gut tube leftward 90. Theoretically, even if left-sided Nodal is normal, alterations in the functions of other factors in or near the gut progenitors could change the asymmetric character of the gut progenitors, leading to an LR asymmetric patterning defect. Indeed, during gut asymmetric migration, Hand2 directly regulates the laminin and matrix metalloproteinase (MMP) activity that is involved in gut asymmetric patterning, however this is not done via Nodal in the LPM 91. Therefore, multiple factors, besides Nodal signaling in the LPM, are involved in regulating gut LR asymmetric morphogenesis.
Early evidence shows that an organ laterality defect can be independent of Nodal
Situations in which organ progenitors and the surrounding cells' characteristics play a critical role in organ LR patterning indicate that organ LR laterality defects may be independent of Nodal in the LPM. This is also supported by some earlier reports. Nodal is necessary for the expression of lefty1, lefty2, pitx2, and bmp4, which are the critical direct regulators of organ LR patterning, however even when the activity of Nodal was normal, the expression of lefty2 could still be disrupted 23, 38, and when Nodal function was lost, lefty2 expression could still be induced 92, 93 and pitx2 expression could be activated via Notch signaling 94, 95. In addition, when nodal was activated bilaterally (in ntl) or was absent (in abf, LZoep, or MZoep) in the diencephalon, habenula laterality could still be established. Therefore, organ LR asymmetric defects are not always the result of abnormal Nodal activity in the LPM (Fig. 4), and different types of organ LR patterning can be uncoupled in some cases.
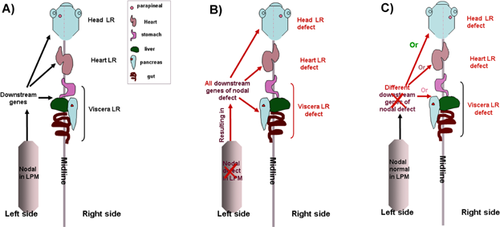
Different types of organ LR patterning can be uncoupled
In some cases, multiple-organ LR patterning was disturbed simultaneously with disruption of the same molecular signaling. In other words, the LR asymmetry of the different organs was determined in a coupled way. Yet, as discussed above, organ LR asymmetric morphogenesis is regulated both by asymmetric Nodal in the LPM and by other factors that directly regulate the characteristics of the organ progenitors or the surrounding cells 23, 24. In addition, for normal development, the duodenum and lungs require a higher dose of pitx2c than the cardiac atria 96. Thus, in this case, the different types of organ LR patterning can be uncoupled, meaning that distinct LR patterning defects for diverse organs can occur when the same signaling is disturbed. Indeed, the misexpression of shh on the right side of the zebrafish embryo reversed the asymmetric gene expression pattern in both the heart and the viscera precursors. In this case, 24% of the embryos injected with 50 pg of shh mRNA on the right side appeared to have reversed hearts, while the viscera developed on the right in only 15% of the injected embryos 97. This indicates that the LR asymmetry patterning of the heart is discordant with that of the gut 97. Furthermore, heart reversals in control embryos are not always accompanied by corresponding asymmetry shifts in the viscera, further demonstrating that there are normal fluctuations in local mechanisms that control asymmetry 97. This uncoordinated organ LR patterning has also been observed in other zebrafish mutants with midline defects (floating head, bozozok, and no tail) 98. Recently, Six3 was reported to specifically regulate neuron LR asymmetric patterning in the zebrafish head 99. In addition, blocking the activity of RA or DeltaD in zebrafish led to a digestive organ laterality defect, but had no effect on LR patterning of the heart 37, 100. So, although different types of organ laterality are regulated by a conserved mechanism 2, 21, 28, 33, 79, individual organ LR patterning can be regulated by individual, distinct mechanisms.
Head and internal organ LR asymmetric development is uncoupled
Recently, the uncoupled nature of laterality for the head and internal organs was reported and the underlying mechanisms were elucidated. Early studies demonstrated that neurological and behavioral asymmetries did not completely correlate with visceral asymmetry 101, 102, though the mechanism for this was unknown. In the early study, Mercola and Levin only postulated that the determination of head laterality might be linked to a head organizer 103. Recently, the precise mechanism underlying the LR asymmetry of the head was elucidated 74, 99, 104, leading to a more complete understanding regarding the uncoupling of head neural and visceral organ laterality. In zebrafish masterblind (mbl) mutants, a mutation in Axin1 disrupts its binding to GSK3β, leading to mild overactivation of Wnt/β-catenin in the anterior neural plate 105. This causes bilateral activation of the Nodal pathway in the epithalamus, but not in the LPM 104. Therefore, only the head asymmetry is disturbed in mbl mutants. The uncoupling of head and internal organ laterality via overactivation of Wnt/β-catenin signaling has also been observed in apc mutants 24, but not in GSK3β morphants, where heart laterality was also disrupted 106. The inconsistent organ LR patterning in mbl and apc mutants can be explained by the assumption that the moderate overactivation of Wnt/β-catenin is sufficient to disrupt Nodal in the epithalamus, but does not change Nodal asymmetry in the LPM either directly or indirectly, and therefore does not disrupt the normal internal organ laterality. This assumption was further supported by the fact that greatly increasing the activity of the Wnt signal by injecting wnt3 mRNA or wnt8 mRNA resulted in abnormal ciliogenesis, Nodal asymmetry and subsequent heart and gut laterality defects 24. As with zebrafish mbl mutants, loss of function in Six3, the repressor of Nodal in the head, results in brain-specific deregulation of Nodal activity and epithalamic laterality defects, however, loss of Six3 function has no effect on visceral LR asymmetric patterning 99. Thus, manipulating the genes that specifically disrupt Nodal activity in the head only affects head asymmetry patterning.
FGF signaling has been reported to be involved in regulating KV/ciliogenesis in zebrafish 39, 40, 107. Loss of this signaling has been found to cause malformation of KV/ciliogenesis and a defective expression pattern of nodal/spaw in the LPM, leading to LR asymmetric defects in the heart, viscera, skeleton, and head. During asymmetric patterning in the head, fgf8 expresses bilaterally with a subtle asymmetry in habenular precursor cells, and there is a mild asymmetric expression from the receptor fgfr4, which expresses much less on the right than on the left 74. In the fgf8 mutant acerebellarti282, the absence of mild asymmetric FGF activity in the epithalamus specifically leads to defective movement of the parapineal nucleus, even though the asymmetric Nodal in the epithalamus and body is normal, as is the viscera laterality 74. In addition, even in the absence of Nodal signaling, fgf8 is sufficient to direct the laterality of parapineal nucleus migration 74. Thus, the result observed in fgf8 mutants is consistent with the hypothesis proposed in a previous study by Wilson et al., whereby the left-sided nodal in the LPM was proposed to indirectly influence Nodal signaling in the epithalamus 108. In conclusion, a specific asymmetric gene expression abnormality in the head can result in a defective parapineal nucleus and habenular LR asymmetric patterning.
Since the number of neurons is regulated by multiple developmental processes such as proliferation, differentiation, migration, and cell death 109, the establishment of habenular LR asymmetry is based on the differences in the number of neurons that belong to each subnucleus between the two sides of the brain 110. Aizawa et al. reported that a common pool of neural stem cells generates the precursors for both the lateral and medial subnuclei, and more precursors of the lateral subnuclei are born on the left side of the habenular nuclei, while more precursors of the medial subnuclei are born on the right side. Further experiments have shown that Notch signaling in the brain specifically determines both the specificity and the asymmetry of neural precursors for the habenular subnuclei at different times, and that neural laterality in the brain does not correlate with laterality in the heart 110, 111.
The above studies imply that LR asymmetry in the head could be regulated by different mechanisms than those regulating the internal organs. Although Nodal in the LPM is critical for asymmetric patterning in the head and the internal organs 30, 112, the specific factors determining the asymmetric patterning of individual organs may play a more direct and crucial role during the establishment of organ asymmetry. Thus, it is conceivable that LR patterning may be uncoupled in the neural system of the head and, in some cases, other body organs.
Uncoupled LR patterning of the heart and digestive organs, and a proposed hypothesis
In most studies, LR patterning defects in the heart and digestive organs were usually found to occur simultaneously. However, some early studies reported that LR patterning defects in the heart and digestive organs were not always caused by the same signaling defect, although the detailed mechanism(s) for this were unclear. It is well known that, after establishing the asymmetric nodal in the LPM, the cardiac and endodermal primordia migrate to the correct locations, and that this is directly regulated by Nodal downstream genes such as pitx2, gata4, and bmp4 24, 33. BMP signals have been reported to be involved in regulating LR patterning in cardiac and digestive organs via divergent mechanisms 77, 113-116. In zebrafish, loss of BMP function at the gastrulation and early somitic stage resulted in abnormal Nodal in the LPM and led to LR asymmetry defects in the heart and digestive organs 23, 115. After 13So, loss of BMP function, using heat-shocking zebrafish tg(hsp70:noggin3) embryos, altered the asymmetric BMP activity mediated by the Bmp4 ligand, leading to a heart LR defect, however, Nodal signaling in the LPM and the digestive organs was not affected 23. Based on these results, Chocron et al. proposed that BMP signaling is involved in regulating LR patterning at two distinct developmental time points. In the early stage, the normal activity of BMP in DFCs and KV represses Nodal in the right LPM to ensure LR patterning in both the heart and the digestive organs, while in the late somitic stage, blocking BMP signaling alters asymmetric BMP activity (such as Bmp4) in the heart field, but not in the asymmetric gene of the gut. Therefore, only heart LR patterning is affected, and digestive organ asymmetry patterning remains normal 23. As with BMP signaling, canonical Wnt signaling in zebrafish has also been reported to influence LR asymmetry in cardiac and digestive organs at different time points via different mechanisms 24, 79. In the early development stage, Wnt/β-catenin signaling is required for KV development, as well as for the regulation of both heart and digestive organ laterality. However, during later asymmetric signal propagation, excessive Wnt/β-catenin signaling by injection of wnt3 mRNA and wnt8 mRNA does not disturb the asymmetric expression pattern of nodal/spaw, but rather inhibits the transmission of asymmetric cues from the LPM to the cardiac field, but not to the gut field. Thus, excessive Wnt/β-catenin signaling disturbs the laterality of the heart but not of the digestive organs 24. Further molecular analysis has identified Gata4 as the downstream target of Wnt/β-catenin signaling in the cardiac field and its involvement in the regulation of lefty2 in the LR patterning of the heart 24.
The above studies show that the disruption of KV morphogenesis or cilia function during the early somitic stage leads to the disturbance of nodal in the LPM and simultaneously results in LR defects of the cardiac and digestive organs. During the later somitic stage, blocking the activity of BMP and Wnt signaling only disturbs some specific factors of the heart progenitor (such as bmp4 and gata4), leading to abnormal heart LR asymmetry. However, in a recent study 37, we found that blocking RA signaling in zebrafish embryos with BMS453 treatment prior to 2So resulted in increased cilia length, no directional node flow and led to the expression of nodal/spaw on both sides of the LPM, but did not change the asymmetric pattern of bmp4. This defective nodal activity led to randomized laterality of the digestive organs, but did not cause serious defects in heart LR patterning 37. This inconsistent organ LR patterning is likely because when RA signaling was blocked before 2So, ciliogenesis and node flow were disturbed and caused bilateral expression of nodal/spaw in the LPM; however, the bilateral nodal/spaw could not disrupt the asymmetric expression pattern of bmp4 because other factors were possibly involved in specifically regulating bmp4, and these factors were not affected when RA activity was blocked (Fig. 5). Thus, even the lateral nodal/spaw was changed in the embryos treated with BMS453. Due to the normal asymmetric expression pattern of bmp4, the heart LR asymmetry patterning remained normal. Similar results were obtained in a study conducted by Lopes et al. They reported that the zebrafish aei/deltaD mutant displayed an LR defect in the digestive organs, but no cardiac LR defect, even though cilia length was shortened, the fluid flow velocity was decreased in the KV and nodal/spaw was expressed in both sides of the LPM 100. They pointed out that in the zebrafish aei mutant, the right-sided nodal/spaw could not reach the heart field to disrupt the normal heart LR asymmetry patterning 100. But this explanation does not account for the consistent LR patterning defect in the cardiac and digestive organs in CaMK-II morphons, β-catenin1 morphants or β-catenin2 morphants, where the expression pattern of nodal/spaw was similar to that of aei mutant embryos but the LR patterning in both the cardiac and digestive organs was disturbed 26, 63. Research done by Lopes et al., as well as our experiments, indicate the possibility that other critical factors besides Nodal exist in the LPM, and that these factors might be specifically involved in regulating LR asymmetry patterning in the heart (Fig. 5). In addition to the above two studies, another study also clinically observed uncoupled LR laterality defect of the heart and viscera in individuals with primary ciliary dyskinesia who developed situs inversus abdominalis without any laterality defect in the heart 117.
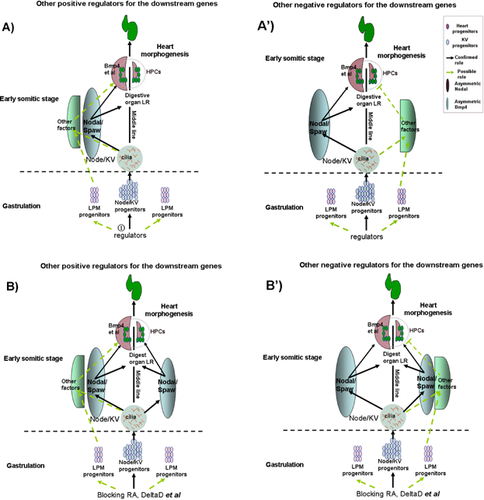
 , yellow-green dotted arrow), and these may contribute to the asymmetric expression of other genes in the left LPM (A, which positively regulates the downstream asymmetric genes) or the right LPM (A′, which negatively regulates the downstream genes). At the early somitic stage, the unidirectional node flow leads to the expression of classic asymmetric Nodal in the left-sided LPM. Normal node function (not node flow) may also induce other asymmetric genes in the left (A) or right (A′) LPM (these possible asymmetric genes only influence some of the specific downstream genes; Bmp4 is an example presented in A and A′). Finally, both Nodal and the possible asymmetric genes regulate the specific downstream genes to control heart LR patterning. In general, most genes reported to be involved in regulating Nodal signaling and the other possible genes that regulate node function or LPM progenitors are committed to this function at the gastrulation stage, so disruption of the function of these genes leads to LR patterning defects in the digestive organ, heart, and neural tissue. B, B′: When RA activity is blocked and the activity of Notch is down-regulated in embryos, only Nodal in the LPM is greatly influenced, while the other possible asymmetric genes are not affected. Because of the redundant role of the genes that possibly regulate the more direct downstream genes for heart LR patterning, it is only laterality of the digestive organs, and not heart laterality, that is affected in the aei mutants and embryos with blocked RA activity.
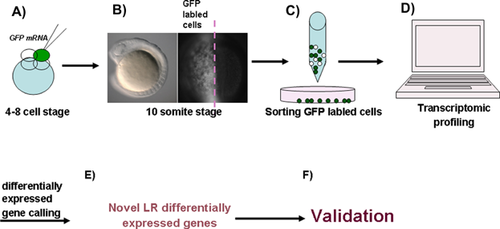
, yellow-green dotted arrow), and these may contribute to the asymmetric expression of other genes in the left LPM (A, which positively regulates the downstream asymmetric genes) or the right LPM (A′, which negatively regulates the downstream genes). At the early somitic stage, the unidirectional node flow leads to the expression of classic asymmetric Nodal in the left-sided LPM. Normal node function (not node flow) may also induce other asymmetric genes in the left (A) or right (A′) LPM (these possible asymmetric genes only influence some of the specific downstream genes; Bmp4 is an example presented in A and A′). Finally, both Nodal and the possible asymmetric genes regulate the specific downstream genes to control heart LR patterning. In general, most genes reported to be involved in regulating Nodal signaling and the other possible genes that regulate node function or LPM progenitors are committed to this function at the gastrulation stage, so disruption of the function of these genes leads to LR patterning defects in the digestive organ, heart, and neural tissue. B, B′: When RA activity is blocked and the activity of Notch is down-regulated in embryos, only Nodal in the LPM is greatly influenced, while the other possible asymmetric genes are not affected. Because of the redundant role of the genes that possibly regulate the more direct downstream genes for heart LR patterning, it is only laterality of the digestive organs, and not heart laterality, that is affected in the aei mutants and embryos with blocked RA activity.These data demonstrate that LR patterning in the heart might be regulated by Nodal signaling and other important factors in the LPM, and that these factors work together to determine heart LR patterning by directly affecting more specific regulators such as bmp4 (Fig. 5). To verify this hypothesis, we plan to experimentally identify the possible critical factors in the LPM, besides Nodal, that are involved in the LR patterning in the near future (see experiment schedule shown in Fig. 6).
Conclusions and outlook
In conclusion, although left-sided Nodal in the LPM is broadly conserved and plays a crucial role in directing organ LR patterning in most vertebrates, there is much evidence to show that asymmetric information is initiated in distinct ways in different research animals 19, 20. At later developmental stages, special asymmetric information 74 and other elements, such as the timing of the specification of different neurons 110, play specific and important roles in the establishment of neural LR asymmetry. Because distinct mechanisms exist for regulating the LR patterning of head, heart, and digestive organs, LR patterning defects in these types of organs usually occur in an uncoupled way. Furthermore, our research concerning the role of RA in LR patterning 37 and the experiments carried out on zebrafish aei mutants 100 have indicated that bilateral activity of Nodal in the LPM does not always result in simultaneous laterality defects in the heart and digestive organs. Therefore, we propose that other critical signals exist, besides Nodal, that coordinate with Nodal in the LPM to specifically regulate LR asymmetry patterning in the heart (Fig. 5). In order to verify this hypothesis, further research is required (see schedule in Fig. 6). Since the reversal of the laterality of whole body organs does not cause functional disorders of the organs, it is important to clarify the specific mechanisms of individual organ LR patterning in order to understand the relationships between LR asymmetry defects in specific organs and functional disorders of organs.
Acknowledgments
We thank Dr. Qiang Guo, Dr. Xiaohong Liu, Dr. Yu Tang, and Dr. Ming Zhang for invaluable discussions and suggestions, and Dr. Xiaoping Yu, Dr. Hsiao Chang Chan, and Dr. Qiang Zhou for their great support. This work was supported by National Natural Science Foundation of China Grant (31201092), Sichuan Province Science and Technology Commission Grant (2013JY0066), Sichuan Provincial Department of Education Commission Grant (11ZA200), and National Basic Research Program of China (2012CB944903).
The authors have declared no conflict of interest.