Ferroptosis and iron mineralization involved in the death and survival of orange-spotted groupers challenged with Pseudomonas plecoglossicida
Abstract
Pseudomonas plecoglossicida (P. plecoglossicida) is a pathogen in aquaculture that causes considerable economic loss. According to artificial infection experiments, the fish were classified into control group, moribund group, and survival group. Compared to the control group, both the moribund group and the survival group of fish had fewer red blood cells (RBCs) and lower oxygen saturation (SaO2). Furthermore, the fish in the survival group has more RBCs and SaO2 compared to the moribund group. The concentrations of total iron, ferrous iron, ferric iron, and mineralized iron in the fish spleen of the moribund and survival groups were lower compared to those of the control group. Additionally, the concentrations of these iron components in the fish spleen of the survival group were higher than those of the moribund group. The results demonstrated that iron mineralization is involved in the survival of fish challenged with P. plecoglossicida. Compared to the control and survival groups, the fish spleen had several distinguishing features in the moribund group, including less reduced glutathione (GSH), higher mitochondrial complex V activity, more lipid peroxidation, and reactive oxygen species, as well as reduced glutathione peroxidase 4 (gpx4) expression. Moreover, there were intact cell membranes, a normal nucleus size, no chromatin concentration, and disappearance of cristae in the mitochondria of the spleens of the moribund group. The characteristics of spleen cells in the moribund group were consistent with ferroptosis, suggesting that ferroptosis was involved in the death of fish challenged with P. plecoglossicida.
INTRODUCTION
Pseudomonas plecoglossicida is a pathogen of fish that causes high lethality and threatens the development of aquaculture [1-4]. Being challenged with P. plecoglossicida, the spleen of the fish had the highest bacterial load and was one of the most damaged tissues [4, 5]. Fish spleens store blood and recycle senescent red blood cells (RBCs) [6]. These RBCs are restricted to blood vessels or red pulp of the spleen. RBCs lyse quickly when extravasating blood vessels or are released from the red pulp of the spleen [7]. Lysed RBCs release hemoglobin and its ferrous ions, which are oxidative and destructive [8]. The spleen of orange-spotted groupers (Epinephelus coioides) was infiltrated with RBCs when infected with P. plecoglossicida [9]. Efficient processing of ferrous ions released by the RBCs may be involved in the response of orange-spotted grouper to P. plecoglossicida.
The organism's iron homeostasis system coordinates the uptake, transport, and transformation of iron [10]. Massive hemolysis releases ferrous ions and causes iron loss, triggering hypoxia [11, 12]. Additionally, cell-free iron that is mineralized and stored in ferritin not only neutralizes the oxidation of ferrous but also aids in the synthesis of RBCs, thus alleviating hypoxia [13]. Lesser mineralized iron was observed at 2 days post infection (dpi), and more mineralized iron was observed at 5 dpi in the spleen of orange-spotted groupers challenged with P. plecoglossicida [9]. The results indicated that iron mineralization may be involved in the response of orange-spotted groupers to P. plecoglossicida.
During infection and inflammation, the upregulated expression of il-6 and hp stimulates macrophages to scavenge iron [14]. Alternatively, the downregulation of tf expression sequesters iron in macrophages [15]. Iron accumulation affects the glutathione metabolism, leading to lipid peroxidation (LPO), generation of reactive oxygen species (ROS), and disappearance of cristae in mitochondria, ultimately leading to cellular ferroptosis [16]. After being challenged with P. plecoglossicida, the fish cells accumulated lipids, and the cristae of mitochondria disappeared [17]. RNA sequencing (RNA-seq) analysis showed that the expression of il-6 and hp was upregulated and that of tf was downregulated [5]. These results indicated that ferroptosis may be involved in the response of orange-spotted groupers to P. plecoglossicida.
In this study, the fish of the control, moribund, and survival groups were compared, and ferroptosis and iron mineralization were demonstrated to be involved in the death and survival of orange-spotted groupers challenged with P. plecoglossicida, respectively.
MATERIALS AND METHODS
Bacterial culture conditions
The pathogenic P. plecoglossicida NZBD9 strain was obtained from Prof. Yan [18]. The bacteria were cultured in tryptic soy broth medium at 28°C. Cells were washed with phosphate buffered saline (PBS), then harvested and diluted with PBS before infection.
Orange-spotted groupers infection
All fish experiments were performed under the recommendations outlined in the “National Institutes of Health Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Ethics Committee of Sun Yat-sen University.
One thousand six hundred and twenty orange-spotted groupers of 12–15 cm were purchased from a fishery company in Yangjiang, Guangdong province, China. All fish were cultured in circulating water systems for 2 weeks, and specific primers were used to confirm the fish are not infected by P. plecoglossicida [19].
For the survival assay, one hundred and eighty fish were selected randomly for a group. Ninety out of the 180 fish were challenged with 106 colony forming units (cfu) of P. plecoglossicida per fish [9], and the other 90 fish were injected with PBS. The infection lasted 10 days. The survival percent was recorded every 24 h, and the dead fish were removed in time.
In addition to the survival assay, infection experiments were performed 12 times. In each infection experiment, 120 fish were injected, 20 fish were injected with PBS, and 100 fish were injected with P. plecoglossicida. These 12 experiments were used for spleen bacterial counting, RBC (RBC) counting, serum ROS determination, oxygen saturation (SaO2) determination, histological investigation, spleen iron concentration determination, spleen gene expression quantification, spleen mitochondrial complex V activity determination, spleen ROS determination, spleen LPO determination, spleen glutathione determination, and spleen cell ultrathin section observation. The spleens of the fish, which were used to detect the number of RBCs, serum ROS, and SaO2, were used for weighing.
Histopathological investigation
The spleens of the control group, the moribund group, or the survival groups were embedded in paraffin and sectioned after being fixed in Formalin-Aceto-Alcohol fix solution (Coolaber, China). The sections were stained with hematoxylin and eosin (H&E), Van Gieson (VG), and potassium hexacyanoferrate (II) respectively as previously described [20]. H&E was used to stain cytoplasm and nucleus; VG was used to stain collagen fibers; potassium hexacyanoferrate (II) was used to stain the mineralized iron stored in ferritin.
Bacterial cell counting and RBC counting
For the bacterial cell counting assay, samples were ground and serial diluted with PBS, and 100 μL of slurry was dispersed evenly on tryptic soy agar (TSA) and cultured in a constant temperature in an incubator at 28°C for 24 h; then counting and calculating the number of colonies.
For the RBC counting assay, 10 μL of blood was diluted with 2 mL of erythrocyte dilution (Saint-Bio, China) before counting with a cell counting plate [21].
Iron quantification
The method of iron quantification was described by Hunter [22]. The spleens of the control group, the moribund group, or the survival groups were mixed with 0.01 mol/L hydrochloric acid (HCl) (1 mg: 10 μL), then homogenized at 4°C, and centrifuged at 845 g for 5 min. The supernatant was diluted by 0.01 mol/L HCl. Total iron was quantified using the Hemoglobin test solution (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's instructions. Fe2+ was quantified using ferrozine. Briefly, 0.01 mol/L ferrozine reaction solution was prepared by 0.1 mol/L ammonium acetate. Ferrozine reaction solution (18 μL) was mixed with 180 μL test solution. After 30 min, the OD560 was measured. Fe3+ was calculated using the following formula: Fe3+ = total iron—Fe2+.
Determination of ROS
ROS Assay Kit (Beyotime Biotechnology) was used to determine ROS. The O12 probe was diluted 10 times with ddH2O for later use. Blood and anticoagulant, Alsever's solution (Saint-Bio, China), were mixed at a ratio of 1:2, centrifuged at 845 g for 5 min, 100 μL supernatant plasma was mixed with 10 μL diluted O12 probe, incubated at 37°C for 30 min, and the fluorescence intensity was detected (excitation light: 488 nm; emission light: 520 nm). The spleens of the control group, the moribund group, or the survival group were mixed with PBS (1 mg: 10 μL), then homogenized at 4°C and centrifuged at 845 g for 5 min. The 100 μL of supernatant was mixed with 10 μL diluted O12 probe, incubated at 37°C for 30 min, and detected for the fluorescence intensity (excitation light: 488 nm; emission light: 520 nm).
Oxygen saturation determination
The SaO2 calculation method of orange-spotted groupers was described by Sun [9]. Briefly, oxygen saturation was calculated by the following formula: SaO2 = cO2Hb (experiment group)/cHb(experiment group). c(Hb) was measured with a hemoglobin test solution (Nanjing Jiancheng Bioengineering Institute, China). The hemoglobin concentration was calculated as Hb (mol/L) = (ODmeasure - ODblank) × dilution/ε540(Hb). The optical density of blood was acquired by BioTek Synergy Neo2. Subsequently, the cO2Hb was calculated by the formula: c1 = (ε2 × c3)/(ε1 × ε2), and c5 = c4 minus c1, where ε1 : εO2Hb, ε2: εHHb, c1 : cO2Hb (control minus the moribund group/the survival group), c2 : cHHb (control minus the moribund group/the survival group), c3 : cHb (the moribund group/the survival group), c4 : cHb (the control group), and c5 : cO2Hb (the moribund group/the survival group).
Quantitative real-time PCR (qRT-PCR)
RNA was extracted using Eastep Super Total RNA Extraction Kit (Promega, China) according to the manufacturer's instructions. 500 ng RNA were used to perform reverse transcription by Evo M-MLV RT Premix for polymerase chain reaction (PCR) (Accurate Biology, China) according to the manufacturer's instructions. Then, qRT-PCR was carried out using SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biology) according to the manufacturer's instructions. The primers were listed (5′-3′): β-actin, F: GGCTACTCCTTCACCACCACA, R: GGGCAACGGAACCTCTCAT; nramp, F: GGAAGCGGCAGGAAA, R: CAGCAGTCAGCGACAATAA; hcp1, F: TGCTGGGCTACGAGTACGGC, R: GACGGCTTTGCGGATCTTG; fpn1, F: TGTAATAATCGCACTCCACT, R: ACCATCACAATACGCTCAC; tfrc, F: GCGTCCAAACCGAGTC, R: CGTAGTTGCCATACACCAG; steap3, F: GGAAGTCTGCCCGCTAC, R: AAGTTTCACCTCCCTCACC; heph, GGCGATTTCCTTGGG, R: GGCAGCTACACCTACCG; tf, F: AGGAGAATGCGGACAAC, R: AGGCAGAACACTGGGAG; ftl, F: CAGGGCACATTCAAGCG, R: GGGCACCATAAGATACAAAGG; fth1, F: ACTGTGAGGCTGCCATTA, R: GTTCGGTCGCCATTTT; hamp, F: TGCCGCCAGTGAAG, R: GAGTCGGGTAGCAGTAAG; hp, F: GTTCCTCCGTCTCCTTTA, R: CAGTTCTTTCGGTTTGC; hpx, F: CATTGGTAATAGGGTGGGC, R: GGAGGAACTTGGCATTGAG; gpx4, TCCCACGAAGCCAGAA, R: GGGCAAAGGGCAACT.
Determination of fish mitochondrial complex V activity
Fish Complex V Elisa Kit (Abmart, China) was used to determine the mitochondrial complex V activity of orange-spotted groupers' spleen. The spleens of the control group, the moribund group, or the survival group were mixed with PBS (1 mg: 10 μL), then homogenized at 4°C and centrifuged at 845 g for 5 min. Mixing 10 μL supernatant, 40 μL sample diluent buffer and 100 μL HRP-conjugate reagent. The mixture was incubated at 37°C for 60 min. After discarding the liquid, 50 μL of chromogen solution A and chromogen solution B were added, incubated in dark at 37°C for 15 min. The OD450 was detected after adding 50 μL of stop solution.
Determination of LPO
LPO Assay Kit (Nanjing jiancheng) was used to determine LPO of orange-spotted groupers' spleen. The spleens of the control group, the moribund group or the survival group were mixed with PBS (1 mg: 10 μL), homogenized at 4°C, and centrifuged, at 845 g for 10 min. After mixing 200 μL of supernatant with 650 μL of buffer 1, 150 μL of buffer 2 was added. The mixture was incubated at 45°C for 60 min, and then centrifuged at 1500 g for 10 min. The mixture was diluted with absolute ethanol, 200 μL of diluent was used to detect the OD586.
Determination of reduced glutathione (GSH) and oxidized glutathione disulfide (GSSG)
GSH and GSSG Assay Kit (Beyotime Biotechnology, China) was used to determine GSH and GSSG of orange-spotted groupers' spleen. The spleens of the control group, the moribund group, or the survival groups were mixed with PBS (1 mg: 10 μL), then homogenized at 4°C and centrifuged at 845 g for 5 min. The supernatant was used to detect GSH and GSSG levels according to the manufacturer's protocol. Reduced GSH concentration was calculated using the following formula: [GSH] = [total GSH] − 2 × [GSSG].
Transmission electron microscopy
The spleens of the control group, the moribund group or the survival group were cut up, pushed through a 100-mesh nylon screen, and washed twice by PBS. After centrifuging at 400 g for 10 min at 4°C, the cell sediment was fixed by 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 4 h. The fixed sample was used to make ultra-thin slices, and observed the slices through a transmission electron microscope.
Statistical analyses
All data are expressed as the means ± standard deviation from at least three sets of independent experiments. Date analysis was carried out by SPSS 26.0 software (Chicago, IL, USA), and one-way analysis of variance with Dunnett's test was used. p value < 0.05 was considered statistically significant. The method of 2−△△Ct was used to calculate the relative level of gene expression.
RESULTS
The survival rate of orange-spotted groupers challenged with P. plecoglossicida
A total of one hundred and eighty 12–15 cm orange-spotted groupers were randomly selected; 90 fish were divided into a group and injected with 106 cfu P. plecoglossicida (experimental group) or an equal volume of PBS (control group). Then, 30 fish were raised in an independent circulating water system. No fish died in the control group. In the experimental group, the survival percent was 100% at 1 day post infection (dpi), 71.11% at 2 dpi, 35.56% at 3 dpi, 18.89% at 4 dpi, 10% at 5 dpi, and 6.67% at 6 dpi, and no more deaths occurred at 7 dpi (Table 1). The average survival rate was 6.67%. Accordingly, the moribund fish at 2 dpi were regarded as the moribund group, the surviving fish at 7 dpi were regarded as the survival group, and the fish injected with PBS were regarded as the control group.
| 1 dpi | 2 dpi | 3 dpi | 4 dpi | 5 dpi | 6 dpi | 7 dpi | 8 dpi | 9 dpi | 10 dpi | Survival percent | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBS | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100% |
| PBS | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100% |
| PBS | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 100% |
| PP | 30 | 21 | 8 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 3.33% |
| PP | 30 | 21 | 12 | 6 | 4 | 2 | 2 | 2 | 2 | 2 | 6.67% |
| PP | 30 | 22 | 12 | 8 | 4 | 3 | 3 | 3 | 3 | 3 | 10% |
- Abbreviations: dpi, days post infection; PBS, challenging with phosphate buffered saline; PP, challenging with P. plecoglossicida.
Physiological characteristics in the control, moribund, and surviving groups
In the control group, the average spleen mass was 0.0240 g, the bacterial load in the spleen was 0, and the number of RBCs was 5.85 × 106 cells/mm3 (Figure 1A–C). In the moribund group, the average spleen mass was 0.0311 g, the bacterial load in the spleen was 1.39 × 107 cfu/g, and the number of RBCs was 1.82 × 106 cells/mm3 (Figure 1A–C). In the survival group, the average spleen mass was 0.0265 g, the bacterial load in the spleen was 1.69 × 105 cfu/g, and the number of RBCs was 3.16 × 106 cells/mm3 (Figure 1A–C). Compared with the control group (standardized as 1), the serum ROS level was 1.57 in the moribund group and 0.50 in the survival group (Figure 1D); the level of SaO2 was 51.96% in the moribund group and 85.40% in the survival group (Figure 1E). These results indicated that the infection resulted in changes in the number of RBCs, serum ROS and level of SaO2 in orange-spotted groupers. A greater reduction in SaO2 and the number of RBCs, as well as more serum ROS were identified in dead fish, and a smaller reduction in SaO2 and the number of RBCs, as well as less serum ROS were identified in surviving fish.

Physiological differences between control, moribund, and surviving groups. (A) Spleen mass. n = 10. (B) Bacterial load of spleen. n = 3. (C) The serum reactive oxygen species level. n = 3. (D) The number of red blood cells. n = 3. (E) The level of SaO2. n = 3. n.s, no significance. ***p < 0.05.
Histological characteristics of spleen in the control, moribund, and surviving groups
Three fish were randomly selected from each of the control group, the moribund group, and the survival group, and the spleens were sampled and observed. Collagen fibers are important components of blood vessels [23] and are stained red by VG [20]. Collagen becomes granulated, striped or blocky, and blurred boundaries with the surrounding tissue, which is called fibrinoid necrosis [20]. Through observation, it was found that fibrinoid necrosis occurred in the spleen of the moribund group (Figure 2B), but not in the control group nor the survival group (Figure 2A,C). Besides, the spleens were not infiltrated by RBCs in the control group (Figure 2D) and were considerably infiltrated by RBCs in the moribund group (Figure 2E). The spleens of the survival group were not infiltrated by RBCs, but many brown particles accumulated (Figure 2F). The mineralized iron stored in ferritin was abundant and naturally distributed in the spleen of the control group (Figure 2G) but was scarce in the spleen of the moribund group (Figure 2H). In the spleen of the survival group, the mineralized iron stored in ferritin was abundant but less abundant than that of the control group, and it was tightly aggregated (Figure 2I). Mineralized iron stored in ferritin was stained blue after reacting with acidic potassium ferrocyanide (K₄Fe(CN)₆), and the reaction formula is K+ + Fe3+ + [FeII(CN)6]4 → KFeIII[FeII(CN)6] [24]. However, some ferric iron stored in ferritins was stained blue, and some was stained brown (Figure 2I). The reason may be that when iron is in excess, the following reaction will take place: 4Fe3+ + 3[FeII (CN)6]4 → FeIII[FeIIIFeII (CN)6]3, which is brown [24]. The results indicated that the infection resulted in a reduction in mineralized iron stored in ferritin, and little mineralized iron stored in ferritin was involved in fish death. Mineralized iron stored in ferritin was reduced less, and aggregation was involved in fish survival.
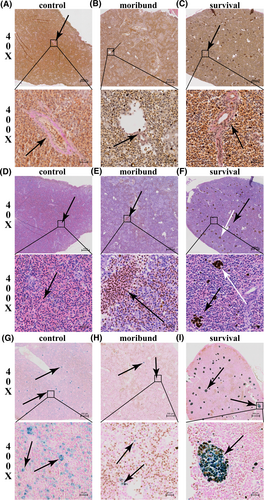
Histological characteristics of spleen in the control, moribund, and surviving groups. (A–C) Van Gieson staining. Collagen fibers are red (arrows). (D–F) H&E staining. Red blood cells aggregation (black arrows), and brown particles aggregation (white arrows). (G–I), iron staining. Ferritin was stained blue (arrow). (A, D, G), the control group; (B, E, H), the moribund group; (C, F, I), the survival group.
Infection caused iron loss in the spleen of orange-spotted groupers
Iron was quantified to investigate whether the reduced mineralized iron was converted to other valances. In the control group, the total iron concentration was 1067.65 μg/g, the ferrous concentration was 380.10 μg/g, the ferric concentration was 687.55 μg/g, the ratio of ferrous/total iron was 36.09%, and the ratio of ferric/total iron was 63.91% (Figure 3A–E). In the moribund group, the total iron concentration was 140.98 μg/g, the ferrous concentration was 102.35 μg/g, the ferric concentration was 38.63 μg/g, the ratio of ferrous/total iron was 74.92%, and the ratio of ferric/total iron was 25.08% (Figure 3A–E). In the survival group, the total iron concentration was 383.32 μg/g, the ferrous concentration was 243.32 μg/g, the ferric concentration was 139.99 μg/g, the ratio of ferrous/total iron was 66.73%, and the ratio of ferric/total iron was 33.27% (Figure 3A–E).
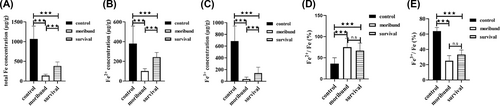
Infection caused iron loss in the spleen of orange-spotted groupers. The level of total iron (A), ferrous (B), ferric (C), ferrous/total iron (D), ferric/total iron (E). n = 3. n.s, no significance. ***p < 0.05.
Compared with the spleen of the control group, the spleen of the moribund group had 926.67 μg/g less total iron, which was 277.75 μg/g less ferrous and 648.92 μg/g less ferric; the spleen of the survival group had 684.33 μg/g less total iron, which was 136.78 μg/g less ferrous and 547.56 μg/g less ferric. Compared with the spleen of the moribund group, the spleen of the survival group had 242.34 μg/g more total iron, which was 140.97 μg/g more ferrous and 101.36 μg/g more ferric.
The above results suggest that infection resulted in a reduction in total iron concentration, ferrous concentration, and ferric concentration, a higher ratio of ferrous/total iron, and a lower ratio of ferric/total iron in the spleen of orange-spotted groupers. However, the reduction of total iron concentration, ferrous concentration and ferric concentration in the spleen of the surviving group was less than that of the moribund group. These results suggest that the infection caused iron loss, and the amount of iron loss correlated with fish death and survival. More total iron concentration, ferrous concentration, and ferric concentration reduced in the spleen were involved in fish death, and lower total iron concentration, ferrous concentration and ferric concentration reduced in the spleen resulted in fish survival.
The expression of iron-related genes
To further understand the metabolism of iron in the spleen, the expression of iron-related genes was measured, and the data were standardized by log2. Steap3 reduces ferric ions to ferrous ions [25]. Heph oxidizes ferrous ions to ferric ions [26]. Compared to the control group, the expression level of steap3 was downregulated and heph was upregulated in the moribund group, indicating that ferric ions were reduced to ferrous ions (Figure 4A). Compared to the control group or the moribund group, the expression levels of steap3 and heph were downregulated in the survival group, indicating that the conversion between ferrous iron and ferric iron was reduced (Figure 4A,B).
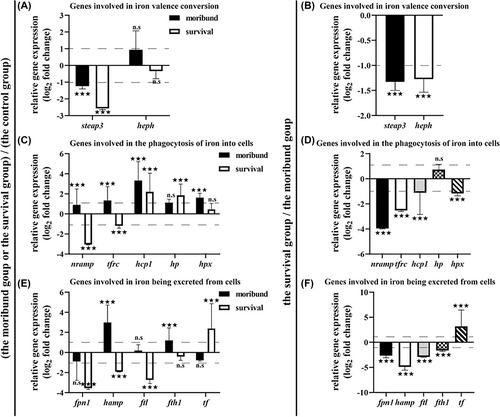
The expression levels of iron-related genes. (A, B), The expression levels of steap3 and heph. (C, D), The expression levels of nramp, tfrc, hcp1, hp and hpx. (E, F), The expression levels of fpn1, tf, hamp, ftl and fth1. (A, C, E): The moribund group (black) or the survival group (white) compared to the control group. (B, D, F): The survival group compared to the moribund group. n = 3. n.s, no significance. ***p < 0.05.
Nramp transports divalent metal ions [27]. Tfrc is a receptor that mediates transferrin entry into cells [28]. Hcp1 transports heme into cells [29]. Hp and Hpx bind cell-free hemoglobin and cell-free heme, respectively, and transport them into macrophages [30, 31]. Compared to the control group, the expression levels of nramp, tfrc, hcp1, hp and hpx were upregulated in the moribund group, indicating that more transferrin, cell-free heme and cell-free hemoglobin were transported into cells; the expression levels of nramp and tfrc were downregulated and hcp1, hp, and hpx were upregulated in the survival group, indicating that divalent metal ions were reduced, less transferrin was transferred into cells and more cell-free heme or cell-free hemoglobin was transferred into cells (Figure 4C). Compared to the moribund group, the expression levels of nramp, tfrc, hcp1, and hpx were downregulated and hp was upregulated in the moribund group, indicating that divalent metal ions were reduced, less transferrin and cell-free heme were transferred into cells, and more cell-free hemoglobin was transported into cells (Figure 4D).
Fpn1 is the only iron efflux protein [32]; Hamp prevents iron efflux, leading to iron accumulation in cells [33]; Ftl is the light chain of ferritin, which assists in the formation of the globular structure, and Fth1 is the heavy chain of ferritin, which is responsible for oxidizing ferrous into ferric, both of which are responsible for storing iron [34]; Tf is transferrin, which is responsible for transporting iron to necessary positions [35]. Compared to those in the control group, the expression levels of fpn1 and tf were downregulated and those of hamp, ftl, and fth1 were upregulated in the moribund group, indicating that iron was confined in the cell and that ferritin synthesis increased (Figure 4E). Compared to the control group and the moribund group, the expression levels of fpn1, hamp, ftl, and fth1 were downregulated and tf was upregulated in the survival group, indicating that iron was exported out of the cell and transferred by transferrin and that ferritin synthesis was reduced (Figure 4E,F).
From the above results, the mechanism of iron metabolism in the spleen of the orange-spotted groupers may be attributed to more iron were transferred into cells and confined in the cells where it was not exported, and with ferritin synthesis increased, which was involved in fish death; when less iron was transferred into cells, intracellular iron was exported out of cells and transferred by transferrin, and ferritin synthesis was reduced, which was involved in fish survival.
Ferroptosis of spleen cells in the moribund group
The gene expression results suggested that iron was phagocytosed into cells and increased the ferritin synthesis in the moribund group (Figure 4E); however, corresponding results were not observed on histology (Figure 2H). Intracellular iron should be mineralized before it can be stored in ferritin [36]. Excess unmineralized ferrous ions in cells will result in ferroptosis [16].
Ferroptosis is characterized by the reduction or disappearance of mitochondrial cristae, an intact cell membrane, a normal nuclear size, no chromatin concentration, decreased GSH, decreased GPX4 activity, LPO accumulation, and the production of large amounts of ROS [16]. P. plecoglossicida infection resulted in the downregulation of gpx4 expression (Figure 5A), higher mitochondrial complex V activity (Figure 5B), LPO accumulation (Figure 5D), less GSH and GSH/GSH + GSSG (Figure 5E), both in the moribund group and in the survival group, and more spleen ROS accumulation in the moribund group (Figure 5C). Compared to the moribund group, the expression of gpx4 was downregulated (Figure 5A), mitochondrial complex V activity was lower (Figure 5B), spleen ROS levels were lower (Figure 5C), LPO levels were lower (Figure 5D), and GSH and GSH/GSH + GSSG levels were higher in the survival group (Figure 5E). Morphologically, there were intact cell membranes, normal nuclear sizes, homogeneous chromatin and abundant cristae in mitochondria in the control group and the survival group (Figure 5F,H). Although some cells had intact membranes and normal-sized nuclei, these cells had no chromatin concentration and absent cristae in mitochondria in the moribund group (Figure 5G).
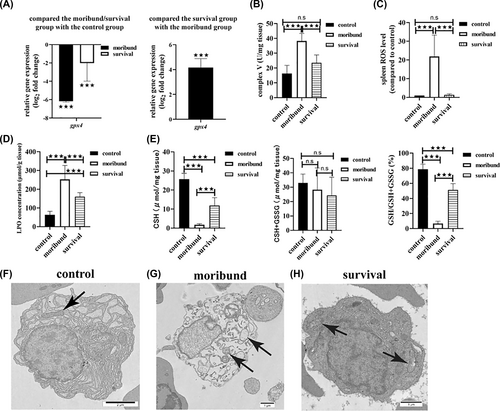
Spleen cells ferroptosis in the moribund group. (A) The expression level of gpx4. n = 3. (B) Mitochondrial complex V activity. n = 3. (C) The spleen reactive oxygen species. n = 3. (D) Lipid peroxidation concentration. n = 3. (E) GSH concentration. n = 3. (F–H) Ultrastructure of spleen cells. (F) The control group. (G) The moribund group. (H) The survival group. ***p < 0.05.
In the survival group, spleen ROS did not accumulate, and the morphological characteristics of spleen cells did not conform to the characteristics of ferroptosis, so there was no cell death in the survival group. All characteristics in the moribund group were consistent with the characteristics of ferroptosis, so ferroptosis was involved in fish death.
DISCUSSION
Iron is widely involved in life activities [37], with the majority of iron being utilized by hemoglobin in the form of heme [38]. However, the release of hemoglobin, particularly during infection, can lead to the generation of toxic ROS through Fenton reaction, posing a significant threat to the host's health [8, 39]. In the case of P. plecoglossicida induced infection in orange-spotted groupers, the spleen experiences vascular necrosis (Figure 2B), extracellular leakage of RBCs (Figure 2E), and production of a substantial amount of ROS (Figure 5C). Therefore, addressing the impact of cell-free iron is essential for the orange-spotted grouper's resistance against P. plecoglossicida.
Biologic mineralization refers to the physicochemical process by which organic ions are converted into inorganic crystals and deposited within cells [40]. Iron mineralization involves the conversion of organic ferrous ions into ferric ions stored as ferritin [41]. This process stabilizes iron and prevents it from participating in redox reactions, thereby exhibiting antioxidative properties [36, 42]. Indeed, compared to the moribund group, the survival group exhibited significantly higher ferritin levels and lower ROS in the spleen (Figures 2I and 5C). The differences in iron mineralization capacity may contribute to the varied resistance of orange-spotted groupers to P. plecoglossicida. In addition, iron is also a nutrient required for bacterial proliferation [43]. Mineralization sequesters iron within the cell, which is a host strategy for enforcing nutritional immunity to prevent pathogens from acquiring iron to proliferate [44]. Moreover, mineralized iron serves as a source for erythropoiesis, alleviating anemia [45]. However, our results suggested that iron mineralization is an effective strategy for orange-spotted groupers to respond to P. plecoglossicida.
Excess iron leads to cellular ferroptosis [46]. Previous studies have shown that ferroptosis is related to the pathogenicity of pathogens to mammals [47]. For instance, pLoxA expressed by Pseudomonas aeruginosa, homologous to mammalian ALOX15, causes ferroptosis in human epithelial cells [48]. Death of the host cell further exacerbated functional defects, resulting in a losing battle against the pathogen. P. plecoglossicida disrupted the blood system in the spleen of orange-spotted groupers, providing an ample source of iron from liberated RBCs for ferroptosis (Figure 2D). Infections may accelerate death rates due to an inflammation storm, rendering the host less capable of resisting further attacks by P. plecoglossicida [5]. Moreover, the excessive iron burden weakens the bactericidal ability of macrophages [15]. Under the influence of various factors, orange-spotted groupers struggle to eliminate P. plecoglossicida, ultimately leading to mortality.
Variable-valence iron is endowed with the ability to donate and accept electrons, which makes it participate in almost all biological redox reactions [49]. In living organisms, iron is precisely regulated and corrected to a limited level by a rigorous system [49]. Iron disorder is often associated with diseases, such as infection and cancer [50]. Cellular ferroptosis is a type of cell death caused by iron disorder [51]. In vitro, ferroptosis inhibitors have been shown to potentially help treat ferroptosis [47]. However, the efficacy remains to be further verified. The orange-spotted groupers provided a new approach to responding to iron disorder in infection, which was iron mineralization. Iron mineralization is a critical step in iron metabolism [36]. This study demonstrated that iron mineralization was involved in host defense against bacterial infection. In the future, the antibacterial mechanism of iron mineralization will be further studied to develop specific drugs to treat infection.
AUTHOR CONTRIBUTIONS
Yujia Sun, and Jianguo He conceived and initiated the project. Yujia Sun, carried out the majority experiments and wrote the manuscript. Chuanfu Dong, revised the manuscript. Shaoping Weng, participated in histological and ultrastructural results analysis. Shaoping Weng, and Jianguo He provided funding support and revised the manuscript.
ACKNOWLEDGMENTS
This work was funded by National Key Research and Development Program of China (No. 2022YFE0203900) and The R&D projects in key areas of Guangdong Province (No. 2021B0202040002).
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
ETHICS STATEMENT
All fish experiments were performed under the recommendations outlined in the “National Institutes of Health Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Ethics Committee of Sun Yat-sen University.
Open Research
DATA AVAILABILITY STATEMENT
All data, models, and code generated or used during the study appear in the submitted article.




