Solvent-free oxidation of straight-chain aliphatic primary alcohols by polymer-grafted vanadium complexes
Payal Kachhap
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Contribution: Data curation (lead), Investigation (lead), Validation (equal), Visualization (equal)
Search for more papers by this authorNikita Chaudhary
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Contribution: Conceptualization (supporting), Formal analysis (supporting), Methodology (supporting), Project administration (supporting), Supervision (supporting)
Search for more papers by this authorCorresponding Author
Chanchal Haldar
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Correspondence
Chanchal Haldar, Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad 826004, Jharkhand, India.
Email: [email protected]
Contribution: Conceptualization (lead), Data curation (supporting), Formal analysis (lead), Funding acquisition (lead), Investigation (supporting), Methodology (lead), Project administration (lead), Resources (lead), Software (lead), Supervision (lead), Validation (equal), Visualization (equal)
Search for more papers by this authorPayal Kachhap
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Contribution: Data curation (lead), Investigation (lead), Validation (equal), Visualization (equal)
Search for more papers by this authorNikita Chaudhary
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Contribution: Conceptualization (supporting), Formal analysis (supporting), Methodology (supporting), Project administration (supporting), Supervision (supporting)
Search for more papers by this authorCorresponding Author
Chanchal Haldar
Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad, India
Correspondence
Chanchal Haldar, Department of Chemistry, Indian Institute of Technology (Indian School of Mines), Dhanbad 826004, Jharkhand, India.
Email: [email protected]
Contribution: Conceptualization (lead), Data curation (supporting), Formal analysis (lead), Funding acquisition (lead), Investigation (supporting), Methodology (lead), Project administration (lead), Resources (lead), Software (lead), Supervision (lead), Validation (equal), Visualization (equal)
Search for more papers by this authorFunding information: Government of India, New Delhi, Grant/Award Number: SB/EMEQ-055/2014
Abstract
Oxidovanadium(IV) complexes [VO(tertacac)2] (1), [VO(dipd)2] (2), and [VO(phbd)2] (3) were synthesized by reacting [VO(acac)2] with 2,2,6,6-tetramethyl-3,5-hepatanedione, 1,3-diphenyl-1,3-propanedione, and 1-phenyl-1,3-butanedione, respectively. Imidazole-modified Merrifield resin was used for the heterogenization of complexes 1–3. During the process of heterogenization, the V4+ center in complex 2 converts into V5+, whereas the other two complexes 1 and 3 remain in the oxidovanadium(IV) state in the polymer matrix. Theoretically, calculated IPA values of 1–3 suggest that 2 is prone to oxidation compared with 1 and 3, which was also supported by the absence of EPR lines in 5. Polymer-supported complexes Ps-Im-[VIVO(tertacac)2] (4), Ps-Im-[VVO2(dipd)2] (5), and Ps-Im-[VIVO(phbd)2] (6) were applied for the solvent-free heterogenous oxidation of a series of straight-chain aliphatic alcohols in the presence of H2O2 at 60°C and showed excellent substrate conversion specially for the alcohols with fewer carbon atoms. Higher reaction temperature improves the substrate conversion significantly for the alcohols containing more carbon atoms such as 1-pentanol, 1-hexanol, and 1-heptanol while using optimized reaction conditions. However, alcohols with fewer carbon atoms seem less affected by reaction temperatures higher than the optimized temperature. A decreasing trend in the selectivity(%) of carboxylic acid was observed with increasing carbon atoms among the examined alcohols, whereas the selectivity towards aldehydes increased. The order of efficiency of the supported catalysts is 4 > 6 > 5 in terms of turnover frequency (TOF) values and substrate conversion, further supported by theoretical calculations.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
Supporting Information
| Filename | Description |
|---|---|
| aoc6437-sup-0001-Supporting_materials.docxWord 2007 document , 4.7 MB |
Figure S1. FT-IR spectrum of [VO(tertacac)2] (1) Figure S2. FT-IR spectrum of [VO(dipd)2] (2) Figure S3. FT-IR spectrum of [VO(phbd)2] (3) Figure S4. Spectral changes obtained during stepwise addition of one drop portions of 30% H2O2 (0.06 g, 1.76 mmol) in 10 ml of MeOH to: (A) 25 ml of 1.1 × 10−2 M solution of [VO(tertacac)2] (1) in acetone, (B) 25 ml of 0.34 × 10−2 M solution of [VO(dipd)2] (2) in DMF, (C) 25 ml of 0.5 × 10−2 M solution of [VO(phbd)2] (3) in DMF, and (D) Electronic Spectra of complexes 1–3 Figure S5. (A) UV–vis spectra of 1–3 showing d-d transitions. (B) Electronic spectra of polymer-anchored vanadium complexes 4–6 recorded in Nujol Figure S6. Electronic spectra of fresh and recycled catalysts 4–6 recorded in nujol. Figure S7. ORTEP diagram of [VO(tertacac)2] (1) Figure S8. ORTEP diagram of [VO(dipd)2] (2) Figure S9. ORTEP diagram of [VO(phbd)2] (3) Figure S10. Natural population analysis of: (A) [VO(tertacac)2] (1), (B) [VO(dipd)2] (2) and (C) [VO(phbd)2] (3) Figure S11. MO diagram of vanadium complex [VO(dipd)2] (2) Figure S12. MO diagram of vanadium complex [VO(phbd)2] (3) Figure S13:.SOMO alfa M.O.
Figure S14. Gas-phase optimized molecular structures of (A) [VO(tertacac)2] (1), (B) [VO(dipd)2] (2) and (C) [VO(phbd)2] (3) calculated at DFT/ LANL2DZ # 6-311G + g(d,p) mixed level of theory. Figure S15. Selectivity of alcohol oxidation products by catalyst 5 Figure S16. Selectivity (%) of alcohol oxidation products by catalyst 6 Figure S17. Selectivity of alcohols (1-BuOH to 1-HpOH) oxidation products by catalyst 4 at 60 °C and 100 °C.** -CHO denotes the corresponding aldehyde formed, -COOH denotes the corresponding acid formed, -COOR denotes the corresponding ester formed Figure S18. FT-IR spectrum of recycled Ps-Im-[VIVO(tertacac)2] (4) Figure S19. FT-IR spectrum of recycled Ps-Im-[VVO2(dipd)2] (5) Figure S20. FT-IR spectrum of recycled Ps-Im-[VIVO(phbd)2] (6) Table S1. Selected FT-IR data for oxidovanadium (IV) complexes 1–3 and polymer-anchored complexes 4–6. Table S2. Electronic spectral data of oxidovanadium (IV) complexes 1–3 and polymer anchored vanadium complexes 4–6. Table S3. Geometry optimized parameters for the mononuclear vanadium (IV) complexes computed at DFT/LANL2DZ # 6-311G + g(d,p) level Table S4. Quantum chemical properties estimated at DFT/LANL2DZ # 6-311G + g(d,p) mixed level of theory in water using CPCM solvation model Table S5. Crystal and refinement parameters for the complex 1 Table S6. Crystal and refinement parameters for the complex 2 Table S7. Crystal and refinement parameters for the complex 3 Table S8. Metal content (in %) present in the polymer anchored complexes 4–6 obtained from AAS and EDX. Table S9. A comparative study of the catalytic oxidation of primary aliphatic alcohols with contemporary catalysts available in literature. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1a) S. Mannam, G. Sekar, Tetrahedron Lett. 2008, 49, 2457; b) M. Hunsen, Synthesis 2005, 15, 2487; c) Y. Tashino, H. Togo, Synlett 2004, 11, 2010; d) I. Heilbron, E. R. H. Jones, F. Sondheimer, Chem. Soc 1949, 604; e) B. N. Zope, D. D. Hibbitts, M. Neurock, R. J. Davis, Science 2010, 330, 74; f) G. Zhang, B. L. Scott, R. Wu, L. A. Pete Silks, S. K. Hanson, Inorg. Chem. 2012, 51, 7354; g) C. Bai, A. Li, X. Yao, H. Liu, Y. Li, Green Chem. 2016, 18, 1061; h) N. Gunasekaran, Adv. Synth. Catal. 2015, 357, 1990.
- 2a) M. H. Ardakani, S. Saeednia, P. Iranmanesh, B. Konani, J. Inorg. Organomet. Polym. 2017, 27, 146; b) P. Wang, Z. Dong, Y. Lei, Y. Du, H. Li, H. Yang, Y. Nie, J. Ma, J. Porous Mater. 2013, 20, 277.
- 3Š. Možina, J. Iskra, J. Org. Chem. 2019, 84, 14579.
- 4K. Bijudas, T. D. R. Nair, Curr. Chem. Lett. 2014, 3, 109.
- 5A. Shaabani, A. T. Tabatabaei, F. Hajishaabanha, S. Shaabani, M. Seyyedhamzeh, M. K. Nejad, J. Sulfur Chem. 2018, 39, 367.
- 6G. B. Kovács, N. V. May, P. A. Bombicz, S. Klébert, P. Németh, A. Menyhárd, G. Novodárszki, V. Petrusevski, F. P. Franguelli, J. Magyari, K. Béres, I. M. Szilágyi, L. Kótai, RSC Adv. 2019, 9, 28387.
- 7a) H. Liu, Y. Liu, Y. Li, Z. Tang, H. Jiang, J. Phys. Chem. C 2010, 114, 13362; b) C. Shang, Z. Liu, J. Am. Chem. Soc. 2011, 133, 9938; c) B. Karimi, M. Khorasani, H. Vali, C. Vargas, R. Luque, ACS Catal. 2015, 5, 4189; d) K. Kaizuka, H. Miyamura, S. Kobayashi, J. Am. Chem. Soc. 2010, 132, 15096; e) Q. Wang, X. Cai, Y. Liu, J. Xie, Y. Zhou, J. Wang, Appl. Catal. B. 2016, 189, 242; f) L. Jacobse, S. O. Vink, S. Wijngaarden, L. B. F. Juurlink, J. Chem. Educ. 2017, 94, 1285; g) M. Ketkaew, D. Suttipat, P. Kidkhunthod, T. Witoon, C. Wattanakit, RSC Adv. 2019, 9, 36027; h) C. D. Pina, E. Falletta, M. Rossi, Chem. Soc. Rev. 2012, 41, 350; i) C. V. Doorslaer, Y. Schellekens, P. Mertens, K. Binnemans, D. D. Vos, Phys. Chem. Chem. Phys. 2010, 12, 1741; j) P. Verma, K. Mori, Y. Kuwahara, S. J. Cho, H. Yamashita, Catal. Today 2020, 352, 255; k) S. Yurdakal, B. S. Tek, C. Değirmenci, G. Palmisano, Catal. Today 2017, 281, 53; l) K. M. Waldie, K. R. Flagslik, E. McLoughlin, C. E. D. Chidsey, R. M. Waymouth, J. Am. Chem. Soc. 2017, 139, 738; m) R. Ray, S. Chandra, D. Maiti, G. K. Lahiri, Chem. – Eur. J. 2016, 22, 8814; n) Y. Wang, Z. Wu, H. Yu, S. Han, Y. Wei, Green Chem. 2020, 22, 3150.
- 8Š. Možina, S. Stavber, J. Iskra, Eur. J. Org. Chem. 2017, 2016, 448.
- 9A. G. J. Ligtenbarg, R. Hage, B. L. Feringa, Coord. Chem. Rev. 2003, 237, 89.
- 10J. C. Pessoa, E. Garribba, M. F. A. Santos, T. Santos-Silva, Coord. Chem. Rev. 2015, 301-302, 49.
- 11V. Conte, A. Coletti, B. Floris, G. Licini, C. Zonta, Coord. Chem. Rev. 2011, 255, 2165.
- 12J. A. L. da Silva, J. J. R. F. da Silva, A. J. L. Pombeiro, Coord. Chem. Rev. 2011, 255, 2232.
- 13M. R. Maurya, A. Kumar, J. C. Pessoa, Coord. Chem. Rev. 2011, 255, 2315.
- 14a) M. R. Maurya, C. Haldar, A. A. Khan, A. Azam, A. Salahuddin, A. Kumar, J. C. Pessoa, Eur. J. Inorg. Chem. 2012, 2012, 2560; b) P. Paul, A. Ghosh, S. Chatterjee, A. Bera, S. M. Alam, S. M. Islam, Inorg. Chim. Acta 2019, 492, 198.
- 15a) A. Maurya, A. K. Mahato, N. Chaudhary, N. Kesharwani, P. Kachhap, V. K. Mishra, C. Haldar, Appl. Organometal. Chem. 2020, 34, 1; b) N. Kesharwani, N. Chaudhary, C. Haldar, Catal. Lett. 2021. https://doi.org/10.1007/s10562-021-03594-9 c) T. H. Chuo, R. Boobalan, C. Chen, ChemistrySelect 2016, 1, 2174.
- 16a) F. Sabuzi, E. Churakova, P. Galloni, R. Wever, F. Hollmann, B. Floris, V. Conte, Eur. J. Inorg. Chem. 2015, 2015, 3519; b) S. Kumari, A. K. Mahato, A. Maurya, V. K. Singh, N. Kesharwani, P. Kachhap, I. O. Koshevoy, C. Haldar, New J. Chem. 2017, 41, 13625.
- 17a) M. Sutradhar, L. M. D. R. S. Martins, T. R. Barman, M. L. Kuznetsov, M. F. C. G. da Silva, A. J. L. Pombeiro, New J. Chem. 2019, 43, 17557; b) R. Neumann, M. Levin, J. Org. Chem. 1991, 56, 5707; c) S. Fujibayashi, K. Nakayama, M. Hamamoto, S. Sakaguchi, Y. Nishiyama, Y. Ishii, J. Mol. Catal. A: Chem. 1996, 110, 105; d) Y. Maeda, N. Kakiuchi, S. Matsumura, T. Nishimura, T. Kawamura, S. Uemura, J. Org. Chem. 2002, 67, 6718; e) S. D. Kurbah, M. Asthana, I. Syiemlieh, R. A. Lal, Appl. Organometal. Chem. 2018, 32, 1; f) M. Sutradhar, L. M. D. R. S. Martins, M. F. C. G. da Silvaa, A. J. L. Pombeiro, Appl. Catal. A: Gen 2015, 493, 50; g) D. Kobayashi, S. Kodama, Y. Ishii, Tetrahedron Lett. 2017, 58, 3306; h) L. Rout, P. Nath, T. Punniyamurthy, Adv. Synth. Catal. 2007, 349, 846; i) I. Syiemlieh, M. Asthana, S. D. Kurbah, R. A. Lal, Polyhedron 2019, 170, 202; j) Y. Tang, Z. Du, M. Li, W. Wang, Y. Xiao, Catal. Commun. 2020, 145, 106114; k) M. K. Renuka, V. Gayathri, Catal. Lett. 2019, 149, 1266; l) M. R. Maurya, B. Sarkar, F. Avecilla, I. Correia, Eur. J. Inorg. Chem. 2016, 2016, 4028; m) M. R. Maurya, B. Sarkar, A. Kumar, N. Ribeiro, A. Miliute, J. C. Pessoa, New J. Chem. 2019, 43, 17620.
- 18a) B. N. Wigington, M. L. Drummond, T. R. Cundari, D. L. Thorn, S. K. Hanson, S. L. Scott, Chem. – Eur. J. 2012, 18, 14981; b) S. K. Hanson, R. Wu, L. A. Silks, Org. Lett. 2011, 13, 1908.
- 19a) Y. Khan, T. Kilpiö, M. Marin, V. Russo, J. Lehtonen, R. Karinen, T. Salmi, Chem. Eng. Sci. 2020, 221, 115695; b) R. Li, M. Wu, B. Pan, M. Li, J. Huang, Chem. Res. Chin. Univ. 2020, 36, 934; c) J. Chen, Y. Zhang, D. Zhu, T. Li, Appl. Organometal. Chem. 2019, 34, 1.
- 20M. Peer, N. Weeranoppanant, A. Adamo, Y. Zhang, K. F. Jensen, Org. Process Res. Dev. 2016, 20, 1677.
- 21a) Y. Khan, M. Marin, T. Viinikainen, J. Lehtonen, R. L. Puurunen, R. Karinen, Appl. Catal. A: Gen. 2018, 562, 173; b) Y. Li, D. Nakashima, Y. Ichihashi, S. Nishiyama, S. Tsuruya, Ind. Eng. Chem. Res. 2004, 43, 6021.
- 22a) C. J. Weiss, P. Das, D. L. Miller, M. L. Helm, A. M. Appel, ACS Catal. 2014, 4, 2951; b) C. Ding, W. Luo, J. Zhou, X. Ma, G. Chen, X. Zhou, D. Li, ACS Appl. Mater. Interfaces 2019, 11, 45621; c) T. P. Nicholls, R. A. Bourne, B. N. Nguyen, N. Kapur, C. E. Willans, Inorg. Chem. 2021, 60, 6976; d) A. Neshat, F. Osanlou, M. Kakavand, P. Mastrorilli, E. Schingaro, E. Mesto, S. Todisco, Polyhedron 2021, 193, 114873; e) S. Oudi, A. R. Oveisi, S. Daliran, M. Khajeh, R. Luque, U. Sen, H. García, Appl. Catal. A: Gen. 2021, 611, 117965; f) A. Yoshida, Y. Mori, T. Ikeda, K. Azemoto, S. Naito, Catal. Today 2013, 203, 153.
- 23L. Wang, D. Pan, M. Zhou, Q. Liang, Z. Li, Solid State Sci. 2021, 113, 106546.
- 24a) I. Gandarias, E. Nowicka, B. J. May, S. Alghareed, R. D. Armstrong, P. J. Miedziak, S. H. Taylor, Catal. Sci. Technol. 2016, 6, 4201; b) S. Pordel, M. Rabbani, R. Rahimi, M. Heidari-Golafzani, A. Azad, Solid State Sci. 2020, 107, 106306.
- 25a) H. Tsunoyama, H. Sakurai, Y. Negishi, T. Tsukuda, J. Am. Chem. Soc. 2005, 127, 9374; b) Y. Sawayama, H. Shibahara, Y. Ichihashi, S. Nishiyama, S. Tsuruya, Ind. Eng. Chem. Res. 2006, 45, 8837.
- 26a) H. Ünver, I. Kani, Polyhedron 2017, 134, 257; b) S. D. Kurbah, A. Kumar, I. Syiemlieh, M. Asthana, R. A. Lal, Inorg. Chem. Commun. 2017, 86, 39.
- 27a) D. Sannino, V. Vaiano, P. Ciambelli, G. Carotenuto, M. di Serio, E. Santacesaria, Catal. Today 2013, 209, 159; b) B. Beck, M. Harth, N. G. Hamilton, C. Carrero, J. J. Uhlrich, A. Trunschke, S. Shaikhutdinov, H. Schubert, H.-J. Freund, R. Schlögl, J. Sauer, R. Schomäcker, J. Catal. 2012, 296, 120; c) S. Wang, S. Li, R. Shi, X. Zou, Z. Zhang, G. Fu, L. Li, F. Luo, Dalton Trans. 2020, 49, 2559; d) S. Zavahir, Q. Xiao, S. Sarina, J. Zhao, S. Bottle, M. Wellard, J. Jia, L. Jing, Y. Huang, J. P. Blinco, H. Wu, H.-Y. Zhu, ACS Catal. 2016, 6, 3580; e) A. Kumar, S. D. Kurbah, I. Syiemlieh, S. A. Dhanpat, R. Borthakur, R. A. Lal, Inorg. Chim. Acta 2021, 515, 120068.
- 28R. A. Rowe, M. M. Jones, Inorganic Syntheses, Vol. 5, McGraw Hill Book Co. Inc., New York 1957 113.
- 29U. K. Urs, K. C. Anitha, K. L. Raghunathan, S. A. Shivashankar, W. T. Robinson, T. N. G. Row, Acta Cryst. 2001, E57, m242.
- 30U. Schilde, W. Bansse, E. Ludwig, E. Uhlemann, Z. Kristallogr. 1995, 210, 627.
- 31P. K. Hon, R. L. Belford, C. E. Pfluger, J. Chem. Phys. 1965, 43, 1323.
- 32M. R. Maurya, M. Kumar, A. Arya, Catal. Commun. 2008, 10, 187.
- 33M.J. Frisch, Gaussian 09, Rev. D.01, Gaussian, Inc., Wallingford, CT, 2009.
- 34R.D. Dennington II, T.A. Keith, J.M. Millam, GaussView 5.0, Wallingford, CT, 2009.
- 35I. P. Malkerova, A. M. Makarevich, A. S. Alikhanyan, N. P. Kuz'mina, Russ. J. Inorg. Chem. 2017, 62, 818.
- 36M. Mohamadi, S. Y. Ebrahimipour, M. Torkzadeh-Mahani, S. Foro, A. Akbari, RSC Adv. 2015, 5, 101063.
- 37F. Jiang, O. P. Anderson, S. M. Miller, J. Chen, M. Mahroof-Tahir, D. C. Crans, Inorg. Chem. 1998, 37, 5439.
- 38D. C. Crans, A. R. Khan, M. Mahroof-Tahir, S. Mondal, S. M. Miller, A. la Cour, O. P. Anderson, T. Jakusch, T. Kiss, J. Chem. Soc., Dalton Trans. 2001, 3337.
- 39S. Tangestaninejad, M. H. Habibi, V. Mirkhani, M. Moghadam, G. Grivani, J. Mol. Catal. A: Chem. 2006, 255, 249.
- 40J. Carmona-Espíndola, J. L. Gázquez, A. Vela, S. B. Trickey, J. Phys, Chem. A 2020, 124, 1334.
- 41J. K. Cooper, C. D. Grant, J. Z. Zhang, Rep. Theor. Chem. 2012, 1, 11.
10.2147/RTC.S36686 Google Scholar
- 42a) M. R. Maurya, A. Arya, P. Adão, J. C. Pessoa, Appl. Catal. A: Gen. 2008, 351, 239; b) M. R. Maurya, N. Chaudhary, F. Avecilla, Polyhedron 2014, 67, 436.
- 43a) M. Sarkheil, M. Lashanizadegan, M. Ghiasi, J. Mol. Struct. 2019, 1179, 278; b) H. Ünver, I. Kani, J. Chem. Sci. 2018, 130, 33; c) Y. Kilic, S. Bolat, I. Kani, J. Coord. Chem. 2018, 71, 2293.
- 44N. Kesharwani, N. Chaudhary, C. Haldar, Catal. Today 2021. https://doi.org/10.1016/j.cattod.2021.06.005
- 45S. Murahashi, T. Naota, N. Hirai, J. Org. Chem. 1993, 58, 7318.
- 46a) G. B. Shul'pin, Y. N. Kozlov, G. V. Nizova, G. Süss-Fink, S. Stanislas, A. Kitaygorodskiy, V. S. Kulikova, J. Chem. Soc., Perkin Trans. 2001, 2, 1351; b) Y. N. Kozlov, V. B. Romakh, A. Kitaygorodskiy, P. Buglyó, G. Süss-Fink, G. B. Shul'pin, J. Phys, Chem. A 2007, 111, 7736.
- 47a) X. Li, E. Iglesia, Chem. – Eur. J. 2007, 13, 9324; b) M. Zhao, X.-W. Zhang, C.-D. Wu, ACS Catal. 2017, 7, 6573; c) M. Wang, F. Wang, J. Ma, C. Chen, S. Shi, J. Xu, Chem. Commun. 2013, 49, 6623; d) J. Malineni, H. Keul, M. Möller, Dalton Trans. 2015, 44, 17409.



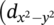 ) of: (A) [VO(tertacac)2] (1), (B) [VO(dipd)2] (2) and (C) [VO(phbd)2] (3)
) of: (A) [VO(tertacac)2] (1), (B) [VO(dipd)2] (2) and (C) [VO(phbd)2] (3)
