Organocatalytic Asymmetric Cycloaddition for the Construction of Chiral Indole-Fused Medium- and Large-Sized Rings
Bo-Wen Lai
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
Search for more papers by this authorHao-Hui Zhang
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P.R. China
Search for more papers by this authorBo-Xuan Yao
Department of Chemistry, Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, and Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, 515063 P.R. China
Search for more papers by this authorRui Li
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Shao-Fei Ni
Department of Chemistry, Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, and Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, 515063 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Kuiyong Dong
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Shi
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P.R. China
Institute of Functional Heterocycles, School of Petrochemical Engineering, Changzhou University, Changzhou, 213164 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorBo-Wen Lai
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
Search for more papers by this authorHao-Hui Zhang
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P.R. China
Search for more papers by this authorBo-Xuan Yao
Department of Chemistry, Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, and Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, 515063 P.R. China
Search for more papers by this authorRui Li
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
Search for more papers by this authorCorresponding Author
Dr. Shao-Fei Ni
Department of Chemistry, Key Laboratory for Preparation and Application of Ordered Structural Materials of Guangdong Province, Shantou University, and Chemistry and Chemical Engineering Guangdong Laboratory, Shantou, 515063 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Kuiyong Dong
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Feng Shi
Research Center of Chiral Functional Heterocycles, School of Chemistry and Materials Science, Jiangsu Normal University, Xuzhou, 221116 P.R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 P.R. China
Institute of Functional Heterocycles, School of Petrochemical Engineering, Changzhou University, Changzhou, 213164 P.R. China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
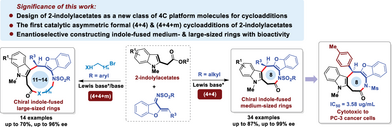
The first catalytic asymmetric (4 + 4) and (4 + 4 + m) cycloadditions of 2-indolylacetates have been established via asymmetric organocatalysis, providing a useful protocol for constructing enantioenriched indole-fused medium- and large-sized rings. The biological evaluation of selected chiral indole products discovered several compounds with some extent of antitumor activity, indicating their potential applications in medicinal chemistry.
Abstract
Catalytic asymmetric construction of chiral indole-fused medium- and large-sized rings represents an important issue in synthetic chemistry but with significant challenges. To overcome these challenges, herein, we report highly enantioselective synthesis of chiral indole-fused medium- and large-sized rings via organocatalytic asymmetric (4 + 4) and (4 + 4 + m) cycloadditions of 2-indolylacetates with benzofuranyl azadienes, providing an efficient strategy for accessing these enantioenriched frameworks. By designing 2-indolylacetates as a new class of four-carbon platform molecules for higher-order cycloadditions and modulating the nucleophilicity of benzofuranyl azadienes, we achieved the catalytic asymmetric synthesis of chiral indole-fused eight-membered rings and eleven- to fourteen-membered macrocycles in high yields with good enantioselectivity. This work not only demonstrates the first application of 2-indolylacetates as four-carbon platform molecules in cycloaddition reactions, but also represents the first catalytic asymmetric (4 + 4) and (4 + 4 + m) cycloadditions of 2-indolylacetates. Furthermore, biological evaluation revealed that several chiral indole derivatives exhibit some extent of antitumor activity, indicating their potential applications in medicinal chemistry.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202507804-sup-0001-SuppMat.pdf15.2 MB | Supporting Information |
| anie202507804-sup-0002-SuppMat.zip554.1 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For some reviews: J.-B. Chen, Y.-X. Jia, Org. Biomol. Chem. 2017, 15, 3550–3567.
- 2P. Milcendeau, N. Sabat, A. Ferry, X. Guinchard, Org. Biomol. Chem. 2020, 18, 6006–6017.
- 3H.-F. Tu, X. Zhang, C. Zheng, M. Zhu, S.-L. You, Nat. Catal. 2018, 1, 601–608.
- 4Y.-C. Zhang, F. Jiang, F. Shi, Acc. Chem. Res. 2020, 53, 425–446.
- 5M.-S. Tu, K.-W. Chen, P. Wu, Y.-C. Zhang, X.-Q. Liu, F. Shi, Org. Chem. Front. 2021, 8, 2643–2672.
- 6H.-H. Zhang, F. Shi, Acc. Chem. Res. 2022, 55, 2562–2580.
- 7B.-M. Yang, X. Q. Ng, Y. Zhao, Chem Catal. 2022, 2, 3048.
- 8H.-H. Zhang, F. Shi, Chin. J. Org. Chem. 2022, 42, 3351.
- 9For some reviews: A. J. Kochanowska-Karamyan, M. T. Hamann, Chem. Rev. 2010, 110, 4489–4497.
- 10M.-L. Luo, W. Huang, H.-P. Zhu, C. Peng, Q. Zhao, B. Han, Biomed. Pharmacother. 2022, 149, 112827.
- 11J. Song, B. Zhang, M. Li, J. Zhang, Fitoterapia 2023, 165, 105430.
- 12For some reviews: Y. Wan, Y. Li, C. Yan, M. Yan, Z. Tang, Eur. J. Med. Chem. 183, 111691.
- 13Y. Hong, Y.-Y. Zhu, Q. He, S.-X. Gu, Bioorg. Med. Chem. 2022, 55, 116597.
- 14M.-L. Luo, Q. Zhao, X.-H. He, X. Xie, H.-P. Zhu, F.-M. You, C. Peng, G. Zhan, W. Huang, Biomed. Pharmacother. 2023, 162, 114574.
- 15For some examples: H. Wang, Z. Bai, T. Jiao, Z. Deng, H. Tong, G. He, Q. Peng, G. Chen, J. Am. Chem. Soc. 2018, 140, 3542.
- 16Z. Bai, S. Zheng, Z. Bai, F. Song, H. Wang, Q. Peng, G. Chen, G. He, ACS Catal. 2019, 9, 6502–6509.
- 17S.-J. Liu, Z.-H. Chen, J.-Y. Chen, S.-F. Ni, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2022, 61, e202112226; Angew. Chem. 2022, 134, e202112226.
- 18M. H. Tse, P. Y. Choy, F. Y. Kwong, Acc. Chem. Res. 2022, 55, 3688–3705.
- 19For recent examples: F. Göricke, C. Schneider, Org. Lett. 2020, 22, 6101–6106.
- 20Q. Li, Y. Zhang, Y. Zeng, Y. Fan, A. Lin, H. Yao, Org. Lett. 2022, 24, 3033.
- 21R. A. Unhale, M. M. Sadhu, V. K. Singh, Org. Lett. 2022, 24, 3319.
- 22Y. Xia, M. Liu, C. Qian, P. Li, M. Dong, W. Li, Org. Chem. Front. 2023, 10, 30.
- 23P. Wu, L. Yu, C.-H. Gao, Q. Cheng, S. Deng, Y. Jiao, W. Tan, F. Shi, Fundam. Res. 2023, 3, 237.
- 24W.-R. Zhu, Q. Su, X.-Y. Deng, Z.-L. Ouyang, J. Weng, G. Lu, Org. Chem. Front. 2024, 11, 2040–2046.
- 25S.-J. Liu, T.-Z. Li, N.-Y. Wang, Q. Cheng, Y. Jiao, Y.-C. Zhang, F. Shi, Org. Chem. Front. 2024, 11, 4812–4819.
- 26L. Hou, L. Yang, G. Yang, Z. Luo, W. Xiao, L. Yang, F. Wang, L.-Z. Gong, X. Liu, W. Cao, X. Feng, J. Am. Chem. Soc. 2024, 146, 23457–23466.
- 27J. Lai, B. List, J. P. Reid, Nat. Commun. 2025, 16, 3676.
- 28For recent examples: H. J. Loui, A. Suneja, C. Schneider, Org. Lett. 2021, 23, 2578–2583.
- 29Y. Tian, F. Wu, S. Jia, X. Gong, H. Mao, P. Wang, W. Qin, H. Yan, Org. Lett. 2022, 24, 5073.
- 30Y.-H. Miao, Z.-X. Zhang, X.-Y. Huang, Y.-Z. Hua, S.-K. Jia, X. Xiao, M.-C. Wang, L.-P. Xu, G.-J. Mei, Chin. Chem. Lett. 2024, 35, 108830.
- 31X. Peng, Y. Huang, W. Wang, S. Li, G.-F. Hao, S. Ren, Y. R. Chi, ACS Catal. 2024, 14, 2127.
- 32T.-Z. Li, S.-J. Liu, S.-F. Wu, Q. Cheng, Q. Chen, Y. Jiao, Y.-C. Zhang, F. Shi, Sci. China. Chem. 2024, 67, 2629–2636.
- 33B.-H. Zhu, Y.-J. Ye, G.-Z. Liu, S.-C. Wu, X. Zou, L. Li, C. Huang, Q. Sun, L.-W. Ye, P.-C. Qian, ACS Catal. 2024, 14, 16639.
- 34J.-Y. Wang, J. Sun, Angew. Chem. Int. Ed. 2025, e20242477; Angew. Chem. 2025, e202424773.
- 35H.-W. Zhao, F. Jiang, S. Chen, J. Hu, S.-H. Xiang, W.-Y. Ding, W. Lu, B. Tan, Angew. Chem. Int. Ed. 2025, 64, e202422951; Angew. Chem. 2025, 137, e202422951.
- 36R. Jiang, N. Wasfy, T. Mori, M. Hoang, I. Abe, H. Renata, Angew. Chem. Int. Ed. 2025, e202502367; Angew. Chem. 2025, e202502367.
- 37J. Barman, S. Ananda, S. C. Pan, Org. Lett. 2025, 27, 1929–1934.
- 38J. Ouyang, R. Maji, M. Leutzsch, B. Mitschke, B. List, J. Am. Chem. Soc. 2022, 144, 8460.
- 39L. Xiao, B. Li, F. Xiao, C. Fu, L. Wei, Y. Dang, X.-Q. Dong, C.-J. Wang, Chem. Sci. 2022, 13, 4801–4812.
- 40K. Balanna, S. Barik, S. Shee, R. G. Gonnade, A. T. Biju, Chem. Sci. 2022, 13, 11513–11518.
- 41J.-Y. Zhang, J.-Y. Chen, C.-H. Gao, L. Yu, S.-F. Ni, W. Tan, F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202305450; Angew. Chem. 2023, 135, e202305450.
- 42B.-Y. Xue, C.-Y. Hou, X.-B. Wang, M.-S. Xie, H.-M. Guo, Org. Chem. Front. 2023, 10, 1910–1914.
- 43D. Liang, P. Gao, Z. Zhang, W. Xiao, J. Chen, G. Synth. Catal 2024, https://doi.org/10.1016/j.gresc.2024.01.002.
10.1016/j.gresc.2024.01.002 Google Scholar
- 44L. Chen, H. Zhou, Y. Xue, L. Kong, Y. Wang, X. Han, H. Yao, A. Lin, ACS Catal. 2024, 14, 8739–8747.
- 45E.-H. Huang, L.-G. Liu, Y.-W. Yin, H.-X. Dong, J.-J. Zhou, X. Lu, B. Zhou, L.-W. Ye, Sci. China Chem. 2024, 67, 2982–2988.
- 46J. Barman, S. Ananda, S. C. Pan, Org. Lett. 2025, 27, 1929.
- 47L. Huang, L.-X. Dai, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5793.
- 48H. Ni, X. Tang, W. Zheng, W. Yao, N. Ullah, Y. Lu, Angew. Chem. Int. Ed. 2017, 56, 14222–14226; Angew. Chem. 2017, 129, 14410.
- 49S. Lu, J.-Y. Ong, H. Yang, S. B. Poh, X. Liew, C. S. D. Seow, M. W. Wong, Y. Zhao, J. Am. Chem. Soc. 2019, 141, 17062–17067.
- 50S. Jia, Y. Tian, X. Li, P. Wang, Y. Lan, H. Yan, Angew. Chem. Int. Ed. 2022, 61, e202206501; Angew. Chem. 2022, 134, e202206501.
- 51Q.-Z. Li, Y.-L. Guan, Q.-W. Huang, T. Qi, P. Xiang, X. Zhang, H.-J. Leng, J.-L. Li, ACS Catal. 2023, 13, 1164.
- 52L.-F. Tao, F. Huang, X. Zhao, L. Qian, J.-Y. Liao, Cell Rep. Phys. Sci. 2023, 4, 101697.
- 53S.-Q. Shi, C.-C. Cui, L.-L. Xu, J.-P. Zhang, W.-J. Hao, J. Wang, B. Jiang, Nat. Commun. 2024, 15, 8474.
- 54X. Lu, Y. Wang, C. He, Y.-Z. Liu, W.-P. Deng, Chin. J. Chem. 2024, 42, 3393–3398.
- 55Z.-X. Zhou, Y.-L.-T. Fu, C. Zhang, Y.-H. Li, B.-J. Zhang, Y.-Q. Xiao, Y.-J. Li, L.-Y. Chen, L. Rao, Y. Tan, W.-J. Xiao, L.-Q. Lu, J. Am. Chem. Soc. 2025, 147, 3223–3232.
- 56G. Yang, Y. He, T. Wang, Z. Li, J. Wang, Angew. Chem. Int. Ed. 2024, 63, e202316739; Angew. Chem. 2024, 136, e202316739.
- 57H.-H. Chen, J.-T. Jiang, Y.-N. Yang, L.-W. Ye, B. Zhou, Angew. Chem. Int. Ed. 2025, 64, e202505167.
- 58H. Zhai, K. Lv, J. Li, J. Wang, T. Liu, C. Zhao, J. Am. Chem. Soc. 2024, 146, 29214–29223.
- 59For a review: S. Zaretsky, A. K. Yudin, in Practical Medicinal Chemistry with Macrocycles: Design, Synthesis, and Case Studies (Eds: E. Marsault, M. L. Peterson), Wiley-VCH, Weinheim, 2017, pp. 1–24.
- 60J. R. Donald, W. P. Unsworth, Chem. - Eur. J. 2017, 23, 8780–8799.
- 61For reviews: V. Martí-Centelles, M. D. Pandey, M. I. Burguete, S. V. Luis, Chem. Rev. 2015, 115, 8736–8834.
- 62K. T. Mortensen, T. J. Osberger, T. A. King, H. F. Sore, D. R. Spring, Chem. Rev. 2019, 119, 10288.
- 63R. L. Reyes, T. Iwai, M. Sawamura, Chem. Rev. 2021, 121, 8926–8947.
- 64For reviews: T. Yao, J. Li, C. Jiang, C. Zhao, Chem. Catal. 2022, 2, 2929.
- 65J. Otevrel, M. Eugui, S. Ričko, K. A. Jørgensen, Nat. Synth. 2023, 2, 1142.
- 66K. Zhu, L. Yang, Y. Yang, Y. Wu, F. Zhang, Chin. Chem. Lett. 2024, 35, 110678.
- 67Z. Sun, H. Tang, L. Wang, D. Cao, Chem. - Eur. J. 2025, 31, e202404217.
- 68B. Riemer, O. Hofer, H. Greger, Phytochemistry 1997, 45, 337–341.
- 69T.-S. Kam, H.-S. Pang, Y.-M. Choo, K. Komiyama, Chem. Biodivers. 2004, 1, 646.
- 70T. Newhouse, C. A. Lewis, P. S. Baran, J. Am. Chem. Soc. 2009, 131, 6360–6361.
- 71K. Kihara, T. Kobayashi, W. Xu, N. Kumagai, Chem. - Eur. J. 2024, 30, e202304176.
- 72For recent reviews: W. Tan, J.-Y. Zhang, C.-H. Gao, F. Shi, Sci. China Chem. 2023, 66, 966.
- 73Z.-Q. Zhu, T.-Z. Li, S.-J. Liu, F. Shi, Org. Chem. Front. 2024, 11, 5573–5604.
- 74For recent examples: Z.-H. Chen, T.-Z. Li, N.-Y. Wang, X.-F. Ma, S.-F. Ni, Y.-C. Zhang, F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202300419; Angew. Chem. 2023, 135, e202300419.
- 75J.-Y. Wang, C.-H. Gao, C. Ma, X.-Y. Wu, S.-F. Ni, W. Tan, F. Shi, Angew. Chem. Int. Ed. 2024, 63, e202316454; Angew. Chem. 2024, 136, e202316454.
- 76P. Wu, W.-T. Zhang, J.-X. Yang, X.-Y. Yu, S.-F. Ni, W. Tan, F. Shi, Angew. Chem. Int. Ed. 2024, 63, e202410581; Angew. Chem. 2024, 136, e202410581.
- 77For early examples: Z.-Q. Rong, M. Wang, C. H. E. Chow, Y. Zhao, Chem. - Eur. J. 2016, 22, 9483–9487.
- 78L.-C. Yang, Z.-Q. Rong, Y.-N. Wang, J. Y. Tan, M. Wang, Y. Zhao, Angew. Chem. Int. Ed. 2017, 56, 2927–2931; Angew. Chem. 2017, 129, 2973.
- 79Z.-Q. Rong, L.-C. Yang, S. Liu, Z. Yu, Y.-N. Wang, Z. Y. Tan, R.-Z. Huang, Y. Lan, Y. Zhao, J. Am. Chem. Soc. 2017, 139, 15304–15307.
- 80Y.-N. Wang, L.-C. Yang, Z.-Q. Rong, T.-L. Liu, R. Liu, Y. Zhao, Angew. Chem. Int. Ed. 2018, 57, 1596; Angew. Chem. 2018, 130, 1612.
- 81For recent examples: Y.-Z. Liu, Z. Wang, Z. Huang, X. Zheng, W.-L. Yang, W.-P. Deng, Angew. Chem. Int. Ed. 2020, 59, 1238; Angew. Chem. 2020, 13, 1254.
- 82X.-X. Yang, R.-J. Yan, G.-Y. Ran, C. Chen, J.-F. Yue, X. Yan, Q. Ouyang, W. Du, Y.-C. Chen, Angew. Chem. Int. Ed. 2021, 60, 26762–26768; Angew. Chem. 2021, 133, 26966.
- 83J. Pan, T. O. Ho, Y.-C. Chen, B.-M. Yang, Y. Zhao, Angew. Chem. Int. Ed. 2024, 63, e202317703; Angew. Chem. 2024, 136, e202317703.
- 84Q. Meng, Y. Meng, Q. Liu, B. Yu, Z.-J. Li, E.-Q. Li, J. Zhang, Adv. Sci. 2024, 11, 2402170.
- 85For recent examples: F. Zhao, C. Shu, C. M. Young, C. Carpenter-Warren, A. M. Z. Slawin, A. D. Smith, Angew. Chem. Int. Ed. 2021, 60, 11892–11900; Angew. Chem. 2021, 133, 11999.
- 86J. Bitai, A. J. Nimmo, A. M. Z. Slawin, A. D. Smith, Angew. Chem. Int. Ed. 2022, 61, e202202621; Angew. Chem. 2022, 134, e202202621.
- 87M. T. Westwood, A. O. Farah, H. B. Wise, M. Sinfield, C. Robichon, M. I. Prindl, D. B. Cordes, P. H.-Y. Cheong, A. D. Smith, Angew. Chem. Int. Ed. 2024, 63, e202407983; Angew. Chem. 2024, 136, e202407983.
- 88Deposition Numbers 2434478 (for 3aa), 2434480 (for 5ara), 2449167 (for 3ar) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.