Enantioselective Divergent Total Syntheses of Cycloaurenones and Dysiherbols
Yu-Hao Huang
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, 310058 China
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorQing-Xiu Gu
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, 310058 China
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorQing-Cen Chao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorHan-Zhi Xiao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorCorresponding Author
Hai-Hua Lu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, 310024 China
E-mail: [email protected]
Search for more papers by this authorYu-Hao Huang
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, 310058 China
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorQing-Xiu Gu
Department of Chemistry, Zhejiang University, 866 Yuhangtang Road, Hangzhou, 310058 China
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorQing-Cen Chao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorHan-Zhi Xiao
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Search for more papers by this authorCorresponding Author
Hai-Hua Lu
Zhejiang Key Laboratory of Precise Synthesis of Functional Molecules, Department of Chemistry, and Research Center for Industries of the Future, Westlake University, 600 Dunyu Road, Hangzhou, 310030 China
Institute of Natural Sciences, Westlake Institute for Advanced Study, 18 Shilongshan Road, Hangzhou, 310024 China
E-mail: [email protected]
Search for more papers by this authorDedicated to Prof. Dr. Wen-Jing Xiao on the occasion of his 60th birthday and to Prof. Dr. Markus Kalesse on the occasion of his retirement
Graphical Abstract
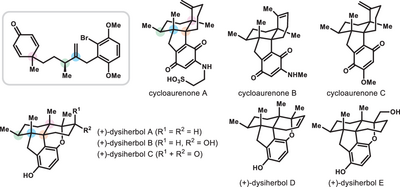
We report the enantiodivergent syntheses of cycloaurenones and dysiherbols via a common cyclohexadienone intermediate, that is, a local desymmetric Giese–Baran-type cyclization and a copper-catalyzed enantioselective conjugate addition reaction. This cyclohexadienone was obtained by a bidirectional synthesis from a chiral bis-Weinreb amide, with stereocenters set by a novel Rh-catalyzed hydrogenation (>99:1 chiral/meso ratio, >99% e.e.).
Abstract
Cycloaurenones and dysiherbols are naturally occurring sesquiterpene quinones/quinols that share a 6/6/5/6 tetracyclic carbon skeleton with either a cis- or trans-decalin system containing four contiguous stereocenters, including three contiguous all-carbon quaternary stereocenters. Total syntheses of cycloaurenones have not been reported. Herein, we present the first enantiodivergent syntheses of cycloaurenones and dysiherbols based on manipulation of a common cyclohexadienone intermediate: namely, a local desymmetric Giese–Baran-type cyclization for cycloaurenones and a copper-catalyzed enantioselective conjugate addition for dysiherbols. Moreover, the key cyclohexadienone intermediate was readily accessible by a bidirectional approach from a chiral bis-Weinreb amide. The 1,4-nonadjacent stereocenters were installed by an unprecedented enantioselective hydrogenation of the corresponding bis-α,β-unsaturated Weinreb amide (>99:1 chiral/meso ratio, >99% enantiomeric excess).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Research data are not shared.
Supporting Information
| Filename | Description |
|---|---|
| anie202507638-sup-0001-SuppMat.pdf21.9 MB | Supporting Information |
| anie202507638-sup-0002-SuppMat.zip732.9 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1X.-H. Tian, L.-L. Hong, W.-H. Jiao, H.-W. Lin, Nat. Prod. Rep. 2023, 40, 718–749, and references therein.
- 2I. S. Marcos, A. Conde, R. F. Moro, P. Basabe, D. Diez, J. G. Urones, Mini-Rev. Org. Chem. 2010, 7, 230–254.
- 3K. Kawashima, K. Nakanishi, H. Nishikawa, Chem. Pharm. Bull. 1964, 12, 796.
- 4W. E. G. Müller, C. Sobel, B. Diehl-Seifert, A. Maidhof, H. C. Schröder, Biochem. Pharmacol. 1987, 36, 1489–1494.
- 5P. S. Sarin, D. Sun, A. Thornton, W. E. G. Müller, J. Natl. Cancer Inst. 1987, 78, 663.
- 6M. Gordaliza, Mar. Drugs 2012, 10, 358–402, and references therein.
- 7A. Rosales Martínez, I. Rodríguez-García, J. L. López-Martínez, Mar. Drugs 2021, 19, 273, and references therein.
- 8B. J. Barras, T. Ling, F. Rivas, Molecules 2024, 29, 279.
- 9T. Ling, A. X. Xiang, E. A. Theodorakis, Angew. Chem. Int. Ed. 1999, 38, 3089–3091.
10.1002/(SICI)1521-3773(19991018)38:20<3089::AID-ANIE3089>3.0.CO;2-W CAS PubMed Web of Science® Google Scholar
- 10T. Ling, E. Poupon, E. J. Rueden, S. H. Kim, E. A. Theodorakis, J. Am. Chem. Soc. 2002, 124, 12261–12267.
- 11H. Ishibashi, K. Ishihara, H. Yamamoto, J. Am. Chem. Soc. 2004, 126, 11122–11123.
- 12J. Sakurai, T. Oguchi, K. Watanabe, H. Abe, S.-i. Kanno, M. Ishikawa, T. Katoh, Chem. - Eur. J. 2008, 14, 829–837.
- 13Y. Fukui, K. Narita, T. Katoh, Chem. - Eur. J. 2014, 20, 2436–2439.
- 14D. D. Dixon, J. W. Lockner, Q. Zhou, P. S. Baran, J. Am. Chem. Soc. 2012, 134, 8432–8435.
- 15B. Schmalzbauer, J. Herrmann, R. Müller, D. Menche, Org. Lett. 2013, 15, 964–967.
- 16K. Speck, R. Wildermuth, T. Magauer, Angew. Chem. Int. Ed. 2016, 55, 14131.
- 17R. Wildermuth, K. Speck, F.-L. Haut, P. Mayer, B. Karge, M. Brönstrup, T. Magauer, Nat. Commun. 2017, 8, 2083.
- 18W. Zhu, Q. Yin, Z. Lou, M. Yang, Nat. Commun. 2022, 13, 6633.
- 19Y. Wu, X. Du, X. Wang, H. Liu, L. Zhou, Y. Tang, D. Li, Org. Chem. Front. 2022, 9, 4705–4711.
- 20Q. Zhang, J. Kang, J. Wang, T. Tan, Z. Lu, Chin. Chem. Lett. 2024, 109915.
- 21C. K. Kim, J. K. Woo, S. H. Kim, E. Cho, Y. J. Lee, H. S. Lee, C. J. Sim, D. C. Oh, K. B. Oh, J. Shin, J. Nat. Prod. 2015, 78, 2814–2821.
- 22A. Eggert, C. Etling, D. Lübken, M. Saxarra, M. Kalesse, Molecules 2020, 25, 3841.
- 23Z. Xin, H. Wang, H. He, S. Gao, Tetrahedron Lett. 2021, 71, 153029.
- 24W. H. Jiao, G. H. Shi, T. T. Xu, G. D. Chen, B. B. Gu, Z. Wang, S. Peng, S. P. Wang, J. Li, B. N. Han, W. Zhang, H.-W. Lin, J. Nat. Prod. 2016, 79, 406–411.
- 25H.-Y. Liu, M. Zhou, R.-Y. Shang, L.-L. Hong, G.-H. Wang, W.-J. Tian, W.-H. Jiao, H.-F. Chen, H.-W. Lin, J. Nat. Med. 2022, 20, 148.
- 26C. Chong, Q. Zhang, J. Ke, H. Zhang, X. Yang, B. Wang, W. Ding, Z. Lu, Angew. Chem. Int. Ed. 2021, 60, 13807.
- 27J. Baars, I. Grimm, D. Blunk, J. M. Neudorfl, H. G. Schmalz, Angew. Chem. Int. Ed. 2021, 60, 14915.
- 28C. Chong, L. Chang, I. Grimm, Q. Zhang, Y. Kuang, B. Wang, J. Kang, W. Liu, J. Baars, Y. Guo, H. G. Schmalz, Z. Lu, Chem. Sci. 2023, 14, 3302.
- 29S. Hu, Y. Tang, J. Am. Chem. Soc. 2022, 144, 19521–19531.
- 30B.-Y. Liu, Z.-C. Zhang, Z.-L. Song, H.-Y. Yuan, Y.-H. Li, Z.-C. Zhang, Z. Yang, Angew. Chem. Int. Ed. 2025, 64, e202415249.
- 31X. Yin, M. Mato, A. M. Echavarren, Angew. Chem. Int. Ed. 2017, 56, 14591.
- 32R. Liu, M. Xia, C. Ling, S. Fu, B. Liu, Org. Lett. 2022, 24, 1642.
- 33M. A. Horwitz, Tetrahedron Lett. 2022, 97, 153776, and references therein.
- 34K. Nakahara, H. Fujioka, Symmetry 2010, 2, 437–454.
- 35S. R. Magnuson, Tetrahedron 1995, 51, 2167–2213.
- 36R. Noyori, Asymmetric Catalysis in Organic Synthesis, Wiley-VCH, New York, 1994.
- 37J. G. de Vries, C. J. Elsevier, The Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, 2007.
- 38Y. Chi, W. Tang, X. Zhang, in Modern Rhodium-Catalyzed Organic Reactions (Ed.: P. A. Evans), Wiley-VCH, Weinheim, 2005, pp. 1–31.
- 39P. Etayo, A. Vidal-Ferran, Chem. Soc. Rev. 2013, 42, 728–754.
- 40H. Yang, H. Yu, I. A. Stolarzewicz, W. Tang, Chem. Rev. 2023, 123, 9397–9446.
- 41N. B. Johnson, I. C. Lennon, P. H. Moran, J. A. Ramsden, Acc. Chem. Res. 2007, 40, 1291–1299.
- 42J. Shang, Z. Han, Y. Li, Z. Wang, K. Ding, Chem. Commun. 2012, 48, 5172.
- 43B. B. C. Peters, N. Birke, L. Massaro, P. G. Andersson, Synlett 34, 2023, 1519–1523.
- 44J. Zhou, J. W. Ogle, Y. Fan, V. Banphavichit (Bee), Y. Zhu, K. Burgess, Chem. - Eur. J. 2007, 13, 7162–7170.
- 45W.-X. Xu, Z. Peng, Q.-X. Gu, Y. Zhu, L.-H. Zhao, H.-H. Lu, Nat. Synth. 2024, 3, 986–997.
- 46G. Xu, C. H. Senanayake, W. Tang, Acc. Chem. Res. 2019, 52, 1101.
- 47H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood IV, J. Aubé, Chem. Rev. 2022, 122, 12544–12747.
- 48K. C. Nicolaou, T. Montagnon, P. S. Baran, Y.-L. Zhong, J. Am. Chem. Soc. 2002, 124, 2245–2258.
- 49T. Mukaiyama, J.-i. Matsuo, H. Kitagawa, Chem. Lett. 2000, 1250.
- 50J. C. Lo, Y. Yabe, P. S. Baran, J. Am. Chem. Soc. 2014, 136, 1304–1307.
- 51A. Simonneau, M. Oestreich, Angew. Chem. Int. Ed. 2015, 54, 3556–3558.
- 52S. L. Shevick, C. V. Wilson, S. Kotesova, D. Kim, P. L. Holland, R. A. Shenvi, Chem. Sci. 2020, 11, 12401–12422.
- 53W. P. Thomas, S. V. Pronin, Acc. Chem. Res. 2021, 54, 1347–1359.
- 54J. Wu, Z. Ma, Org. Chem. Front. 2021, 8, 7050–7076.
- 55S. Mannathan, S. Raoufmoghaddam, J. N. H. Reek, J. G. de Vries, A. J. Minnaard, ChemCatChem 2015, 7, 3923–3927.
- 56For a recent review see: L. J. Oxtoby, J. A. Gurak, Jr, S. R. Wisniewski, M. D. Eastgate, K. M. Engle, Trends Chem. 2019, 1, 572, and references therein.
- 57X. Wu, J. Lei, Z. Song, Chin. Chem. Lett. 2011, 22, 306–309.
- 58P. R. Athawale, V. M. Zade, G. Rama Krishna, D. S. Reddy, Org. Lett. 2021, 23, 6642–6647.
- 59 Deposition numbers 2373960 (for 22), 2407581 (for 2) and 2373958 (for S15) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 60T. L. Liu, T. W. Ng, Y. Zhao, J. Am. Chem. Soc. 2017, 139, 3643–3646.
- 61B. E. Maryanoff, A. B. Reitz, Chem. Rev. 1989, 89, 863–927.
- 62N. A. Petasis, S. P. Lu, E. I. Bzowej, D. K. Fu, J. P. Staszewski, I. Akritopoulou-Zanze, M. A. Patane, Y. H. Hu, Pure Appl. Chem. 1996, 68, 667–670.
- 63F. N. Tebbe, G. W. Parshall, G. S. Reddy, J. Am. Chem. Soc. 1978, 100, 3611–3613.
- 64R. Thompson, E. Nakamaru-Ogiso, C.-H. Chen, M. Pink, D. J. Mindiola, Organometallics 2014, 33, 429.
- 65L. F. Staden, D. Gravestock, D. J. Ager, Chem. Soc. Rev. 2002, 31, 195, and references therein.
- 66K. Takai, Y. Hotta, K. Oshima, H. Nozaki, Tetrahedron Lett. 1978, 19, 2417–2420.
- 67L. Lombardo, Tetrahedron Lett. 1982, 23, 4293–4296.
- 68T.-H. Yan, C.-C. Tsai, C.-T. Chien, C.-C. Cho, P.-C. Huang, Org. Lett. 2004, 6, 4961–4963.
- 69C. Aïssa, R. Riveiros, J. Ragot, A. Fürstner, J. Am. Chem. Soc. 2003, 125, 15512–15520.
- 70A. Krasovskiy, F. K. P. Knochel, Angew. Chem. Int. Ed. 2006, 45, 497.
- 71Y. Zhao, J. Hu, R. Chen, F. Xiong, H. Xie, H. Ding, J. Am. Chem. Soc. 2022, 144, 2495.
- 72R. H., Crabtree, Acc. Chem. Res. 1979, 12, 331–337.
- 73P. A. Grieco, S. Gilman, M. Nishizawa, J. Org. Chem. 1976, 41, 1485–1486.
- 74A. G. Myers, B. Zhang, Tetrahedron Lett. 1996, 37, 4841–4844.
- 75J. Tsuji, I. Minami, I. Shimizu, Synthesis, 1986, 623–627.
- 76C. A. Vincent, V. N. Nair, U. K. Tambar, Org. Lett. 2024, 26, 8453.
- 77D. A. Evans, G. C. Andrews, C. L. Sims, J. Am. Chem. Soc. 1971, 93, 4956–4957.
- 78C. M. Rojas, Molecular Rearrangements in Organic Synthesis; Wiley-VCH, New York, 2015.
10.1002/9781118939901 Google Scholar
- 79C. D. Snyder, H. Rapoport, J. Am. Chem. Soc. 1972, 94, 227–231.
- 80M. Matsuoka, K. Hamano, T. Kitao, K. Takagl, Synthesis 1984, 1984, 953–955.
10.1055/s-1984-31034 Google Scholar
- 81Y. Kuang, L. Chang, B. Wang, J. Kang, C. Chong, Z. Lu, Synlett 2024, 35, 582.
- 82Q. Zhang, Y. Kuang, B. Wang, L. Chang, J. Kang, C. Chong, Z. Lu, Chin. Chem. Lett. 2024, 35, 108338.
- 83W. Yu, P. Hjerrild, K. M. Jacobsen, H. N. Tobiesen, L. Clemmensen, T. B. Poulsen, Angew. Chem. Int. Ed. 2018, 57, 9805–9809.
- 84Y. Hayashi, H. Gotoh, T. Tamura, H. Yamaguchi, R. Masui, M. Shoji, J. Am. Chem. Soc. 2005, 127, 16028–16029.
- 85X.-L. Lu, Y. Qiu, B. Yang, H. He, S. Gao, Chem. Sci. 2021, 12, 4747–4752.
- 86Y. Han, S. Breitler, S.-L. Zheng, E. J. Corey, Org. Lett. 2016, 18, 6172–6175.
- 87Y. Naganawa, M. Kawagishi, J. Ito, H. Nishiyama, Angew. Chem. Int. Ed. 2016, 55, 6873–6876.
- 88A. Bokka, J. X. Mao, J. Hartung, S. R. Martinez, J. A. Simanis, K. Nam, J. Jeon, X. Shen, Org. Lett. 2018, 20, 5158–5162.
- 89Y. Qiao, S. Bai, X.-F. Wu, Y. Yang, H. Meng, J. Ming, Org. Lett. 2022, 24, 1556–1560.
- 90K. A. Kalstabakken, A. M. Harned, Tetrahedron 2014, 70, 9571–9585.
- 91T. H. Al-Tel, V. Srinivasulu, M. Ramanathan, N. C. Soares, A. Sebastian, M. L. Bolognesi, I. A. Abu-Yousef, A. Majdalawieh, Org. Biomol. Chem. 2020, 18, 8526–8571.
- 92T. Shu, J. Cossy, Chem. Soc. Rev. 2021, 50, 658–666.
- 93A. Munakala, M. Phanindrudu, R. Chegondi, Chem. Rec. 2021, 21, 3689–3726.
- 94A. Studer, F. Schleth, Synlett 2005, 3033–3041.
- 95C. Nájera, F. Foubelo, J. M. Sansano, M. Yus, Tetrahedron 2022, 106–107, 132629.
- 96M. C. Willis, J. Chem. Soc. Perkin Tans. 1, 1999, 1765.
10.1039/a906269b Google Scholar
- 97X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou, J. Zhou, Chem. Rev. 2016, 116, 7330–7396.
- 98M. Wang, M. Feng, B. Tang, X. Jiang, Tetrahedron Lett. 2014, 55, 7147–7155.
- 99L. Cala, M. A. Gaviria, S. L. Kim, T. R. Vogel, C. S. Schindler, Synthesis 2023, 55, 1949.
- 100M. Vuagnoux-d'Augustin, A. Alexakis, Chem. - Eur. J. 2007, 13, 9647–9662.
- 101K. Bao, A. Fan, Y. Dai, L. Zhang, W. Zhang, M. Cheng, X. Yao, Org. Biomol. Chem. 2009, 7, 5084.