Xanthopinacol Boronate: A Robust, Photochemically Assembled and Cleavable Boronic Ester for Orthogonal Chemistry
Graphical Abstract
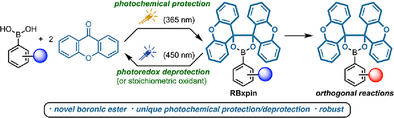
Xanthopinacol (xpin) boronates are a conceptually new class of masked boronic acids designed to alleviate many of the disadvantages of pinacol esters and other popular adducts. They can be formed directly from xanthone and UV light, undergo orthogonal transformations, and are cleavable under photoredox catalysis or oxidative conditions that recycle xanthone to prevent the re-esterification and facilitate the isolation of the boronic acid.
Abstract
Organoboronic acids impact numerous fields of application from organic synthesis to drug discovery and materials science. However, their highly polar nature makes them challenging to handle, purify, and characterize. Boronic esters help overcome these issues, and pinacol esters (Bpin) have become the dominant boronic acid surrogate in organic synthesis. Despite its popularity, Bpin is not without drawbacks. Its formation from pinacol is intrinsically reversible in the presence of water or alcohols, which may cause premature release leading to losses during reactions and purification. This reversibility complicates the hydrolytic regeneration of free boronic acids, which often requires additional steps to destroy the pinacol by-product. Although other boronyl protecting groups exist, their removal requires harsh pH conditions. To address these issues, we developed xanthopinacol boronates (Bxpin), a robust protecting group for boronic acids with excellent orthogonality in various chemical reactions and mild irreversible removal under catalyzed photoredox conditions. Xpin boronates can be obtained directly by irradiation of a mixture of free boronic acid and xanthone with UV light, causing an in situ dimerization of xanthone to the required xanthopinacol. The unique attributes of xpin boronates were further studied by UV–vis spectrophotometry, X-ray crystallography, cyclic voltammetry, and various stability tests.
In the past two decades, organoboronic acids have greatly impacted numerous fields of applications from organic synthesis to drug discovery and materials science.[1-6] Yet due to their highly polar nature and their tendency to form anhydrides, these versatile compounds are notoriously difficult to handle, purify, and characterize. To address these concerns, surrogates of boronic acids, such as their cyclic esters made by a condensation with diols, have been developed to provide good solubility in organic solvents, improved stability toward atmospheric oxidation and protodeboronation,[1] and control of reactivity and selectivity in reactions.[7-11] The low cost of pinacol and the relative stability of the resulting pinacol boronates (Bpin) have led to its status as the dominant boronic acid surrogate. More than 17 000 pinacol boronic esters are commercially available, and more than 150 000 publications use Bpin building blocks or incorporate Bpin into late-stage intermediates.[12, 13] Despite its widespread adoption, Bpin is not without drawbacks. Its formation from pinacol is intrinsically reversible in the presence of water or alcohols,[14-16] which may cause premature release leading to losses from workup or chromatography (Figure 1a). Moreover, pinacol boronates exist mostly as oily materials, they tend to streak on silica TLC and show poor behavior in HPLC analysis.[17, 18] Moreover, due to its reversible behavior, hydrolytic removal to regenerate free boronic acids is complicated and often requires wasteful exchange protocols or methods that destroy the pinacol, such as periodate treatment.[19-22] Many of these and other detriments are documented regularly in large-scale reactions that suffered losses in aqueous work-up,[23] challenging purification by recrystallization,[24] co-precipitation of pinacol by-product from Suzuki–Miyaura cross-coupling,[25] or released pinacol acting as a reaction inhibitor.[26, 27] Alternative protecting groups exist, including the robust MIDA and DAN boronates; however, these derivatives tend to demonstrate a narrow solubility profile and their hydrolytic removal requires harsh acidic or basic aqueous conditions.[28-31] The tetraethyl pinacolate (Bepin) was recently proposed as a robust boronic ester; however, no conditions were developed to regenerate the free boronic acid.[17] These examples highlight a dichotomy in the design of robust boronic esters.[32-40] Whereas sterically hindered or tetracoordinate derivatives offer improved robustness and chemical orthogonality, mild and selective hydrolysis is not possible. To help fill this gap, here we report the design and characterization of xanthopinacol boronates (Bxpin), a photochemically prepared and irreversibly cleavable class of highly soluble boronic esters made from inexpensive xanthone, and their application in orthogonal organic reactions (Figure 1b).[39]
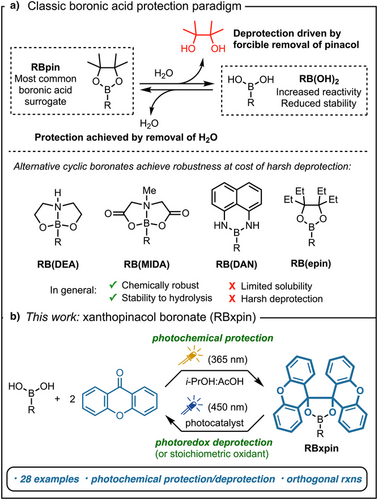
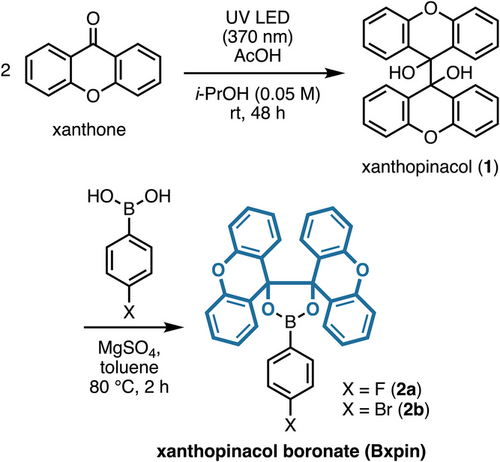
In designing an alternative protecting group to Bpin esters, we sought to mitigate the condensation equilibrium (cf. Figure 1a) whilst retaining a nonpolar, neutral trivalent boron derivative for optimal solubility in organic solvents. This requirement would be met with a hindered diol that can form reversibly under controlled conditions. A photochemical pinacol coupling of biarylketones is ideal for this purpose, and several such reactions have been demonstrated.[41, 42] In selecting a ketone, we were inspired by recent advances in the deprotection of para-methoxybenzyl ethers (including metallophotoredox conditions).[43-46] Therefore, an electron-rich aryl scaffold would offer versatility toward deprotection conditions. A final consideration was the need for an inexpensive, commercially available ketone to encourage ready adoption. Xanthone (<1$ g−1), an easily dimerizable aromatic ketone, emerged as a strong candidate. Thus, xanthopinacol (1) was prepared by the photochemical dimerization of xanthone (Scheme 1).[42] The model xpin boronates 2a and 2b were easily synthesized under dehydrating conditions with anhydrous MgSO4, xanthopinacol (1), and 4-bromo- or 4-fluorophenylboronic acid, yielding bench-stable crystalline products that can be stored for multiple months without the need to exclude air or moisture.
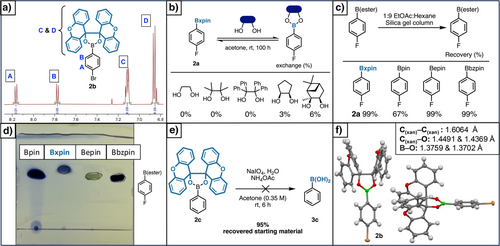
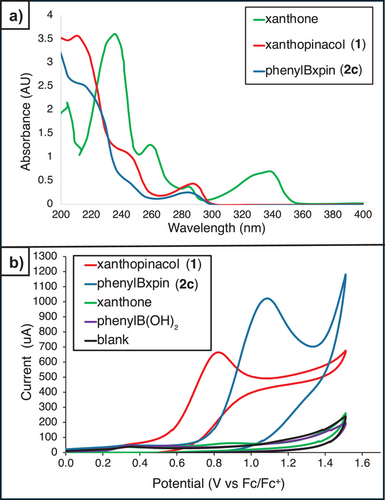
With model ArBxpin esters in hand, the spectrometric and physical properties of this novel boronic acid surrogate were explored. Remarkably, xpin boronate 2b and others in this study were found to be soluble in a wide variety of organic solvents (see Supporting Information: pages S8 and S9). In the 1H NMR analysis in CDCl3 (Figure 2a), 4-bromophenylBxpin (2b) exhibits two well-defined 8H multiplets for the xpin moiety. The relative stability of boronic esters can be assessed through studying their transesterification with other diols.[14-16] Thus, when monitoring the exchange of 2a with pinacol and benzopinacol in acetone-d6, no exchange was observed after more than 100 h (Figure 2b). Pinanediol (6% exchange) is known to form highly thermodynamically stable boronic esters;[14-16] however, its slow exchange with 2a demonstrates the excellent kinetic stability of xpin boronates.[14-16] Furthermore, Bxpin resists a lengthy exposure to water (1 equiv of 2a with 50 equiv of water in acetone-d6 for 139 h) (see pages S10 and S11). Stability toward silica chromatography was assessed by passing 4-fluorophenylboronic esters through a standard column (see pages S7 and S8 for details) and determining the recovery yield (Figure 2c). While only 67% of 4-fluorophenylBpin was recovered, 4-fluorophenylBxpin (2a) was retained quantitatively. This experiment was further confirmed by TLC analysis showing significant baseline breakdown and streaking for the pinacol ester (Figure 2d). Moreover, 2c resists the oxidative aqueous periodate cleavage conditions traditionally employed to revert a Bpin ester to the free boronic acid, which further emphasizes the hydrolytic stability of xpin boronates (Figure 2e). Indeed, Bxpin can tolerate highly acidic conditions (1 equiv of 2a with 10 equiv of TFA in THF for 100 h) and shows good resistance to highly basic conditions (1 equiv of 2a with 10 equiv of NaOH in THF for 100 h; see S11–S13). Structural characterization substantiates the excellent stability of xpin boronates. X-ray crystallography of 2b shows significant steric hindrance around the boron atom (Figure 2f),[47] which undoubtedly hinders transesterification, hydrolysis, and displacement by other nucleophiles, such as amines and carbanions.
A brief optimization of the preparation of xpin boronates was performed using 4-bromophenylboronic acid, xanthopinacol (1), and MgSO4 in toluene (see pages S15 and S16). Initial conditions employed 1.0 equiv of xanthopinacol (1) at 100 °C for 16 h with 5.0 equiv of MgSO4, resulting in an 80% yield of the desired boronic ester. It was found that lowering the temperature to 80 °C and reducing the amount of MgSO4, along with decreasing the reaction time to 2 h, enabled the isolation of 4-bromophenylBxpin (2b) in up to 99% yield even on a gram scale. Reactions without MgSO4 or at room temperature resulted in a decrease of yield and no product, respectively.
Using the optimal conditions, most arylboronic acids examined were readily converted to the corresponding xpin boronates (2a–2x) in excellent yields (Figure 3). Tolerance for several functional groups was demonstrated, including alkenes (2e), esters (2f), and aldehydes (2g). Despite the steric bulk of the Bxpin moiety, ortho-substituted arylboronic acids were successfully protected (2n–2s). Alkenyl (2x) and alkyl boronic acids (2v, 2w) are suitable, affording xpin boronates in high yields. In contrast with the corresponding pinacol boronates, which are not UV-active, aliphatic xpin esters such as 2v and 2w are easily detected under UV light for TLC analysis. A few examples of electron-rich heterocycles, very hindered rings, and acidic substrates were unsuccessful (see page S31).
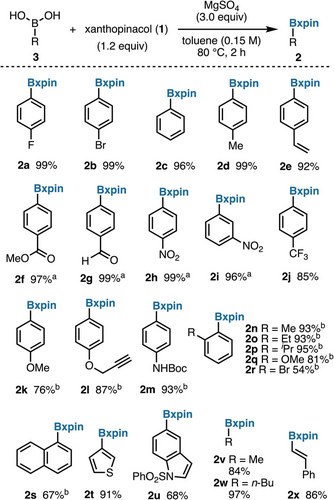
The possibility to protect boronic acids directly from xanthone would provide a convenient, step-economical alternative. Remarkably, introduction of a boronic acid to the photochemical pinacol coupling of xanthone led to high conversion to the corresponding xanthopinacol boronate. As in the two-step protocol, highest yields were achieved with neutral and electron-poor arylboronic acids (Figure 4). Alkenylboronic acids, such as 2x, can isomerize under photochemical conditions,[48, 49] which highlights one limitation of this direct protection procedure.
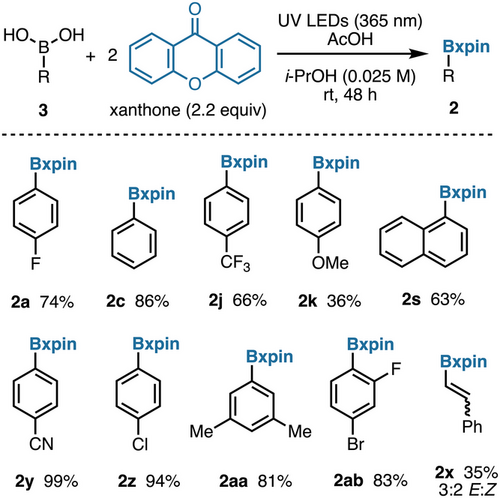
Conditions for cleavage of xpin boronates were probed. UV–vis spectrophotometric analysis shows that xanthone absorbs light near the UV region (Figure 5a), which is in agreement with its effective dimerization in Figure 4. There is no wavelength at which a selective irradiation of an ArBxpin structure could be achieved in the presence of the free xanthopinacol (1) (Figure 5a), whilst cyclic voltammetry (Figure 5b) indicated that irreversible oxidation of both the xanthopinacol and phenylBxpin (2c) is possible at potentials achievable through photocatalysis (see pages S37 and S38 for CV data of other boronates).
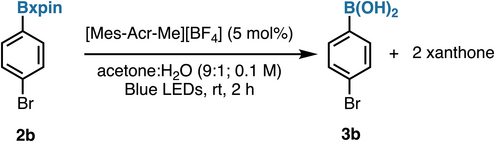
The photoelectrochemical data led us to explore mild photoredox conditions to achieve an irreversible regeneration of boronic acids from xpin esters. This objective would be met by releasing xanthone as opposed to xanthopinacol, thus circumventing the diol condensation equilibrium that often plagues hydrolyses of other boronic esters (cf. Figure 1a). Thus, optimization of solvents (entries 2–4, Table 1), time (entry 5), and catalysts (entries 6–8) confirmed that irradiation with 450 nm LEDs, along with ammonium persulfate (1 equiv)[46] and 9-mesityl-10-methylacridinium tetrafluoroborate (5 mol%) as the photocatalyst in a mixture of acetone and H2O (9:1), maximizes the desired deprotection to the boronic acid. The latter is separated via phase-switching,[50] with xanthone recycled as an easily separable by-product (see pages S39–S42 for additional entries). Moreover, it is worth mentioning that this transformation requires no air-free techniques, as atmospheric oxygen appears to be necessary (see page S42). Preliminary mechanistic studies suggest that the deprotection pathway first involves a slow hydrolysis of Bxpin, releasing a minute amount of the diol, which is subject to photocatalyzed oxidation affording xanthone and the boronic acid (see Supporting Information pages S43–S46 for details). An NMR kinetic profiling experiment was performed and indicated that over time, the generated aryl boronic acid can convert into the corresponding phenol, leading to lower yields (see Supporting Information pages S47–S50 for details). To optimize the yield of boronic acid, four procedures for photochemical deprotection of Bxpin were developed, all relying on the same set of reagents with slight variations (time and photocatalyst loading) (Figure 6). Neutral substrates and substrates bearing electron-withdrawing and -donating substituents were found to be comparable in efficiency, providing the free boronic acid in moderate yields. Nonphotochemical oxidative conditions can provide a complementary alternative for the cleavage of xpin boronates. Thus, use of cerium ammonium nitrate (CAN) in a 1:1 acetonitrile and water mixture efficiently returned the desired boronic acids (Figure 6, Conditions 5).[51] Some substituents are not compatible (e.g., formyl); however, options exist for future improvements, such as electrochemical conditions.
 |
||
| Entrya) | Deviation from equation | Yield (%) |
|---|---|---|
| 1b) | (NH4)2S2O8 (1.0 equiv) | 98 (82) |
| 2 | H2O (5 equiv), PC (20 mol%), acetone, 10 h | 88 |
| 3 | H2O (5 equiv), PC (20 mol%), CH2Cl2, 10 h | 53 |
| 4 | H2O (5 equiv), PC (20 mol%), MeCN, 10 h | 58 |
| 5 | H2O (5 equiv), PC (20 mol%), acetone, 18 h | 63 |
| 6 | H2O (5 equiv), [Ir(dFppy)3 (2.5 mol%), acetone, 10 h | 36 |
| 7 | H2O (5 equiv), [Ir(dFppy)2(dtbbpy)]PF6 (2.5 mol%), acetone, 10 h | 43 |
| 8 | H2O (5 equiv), 4CzIPN (20 mol%), acetone, 10 h | 46 |
| 9 | H2O (5 equiv), PC (20 mol%), acetone, 10 h, no light | Trace |
| 10 | H2O (5 equiv), no PC, acetone, 10 h | Trace |
- a) Unless stated otherwise, reactions were performed on 0.1 mmol scale and yields were determined by 1H NMR analysis using (1,3,5)-trimethoxybenzene as an internal standard, PC = [Mes-Acr-Me][BF4], solvent concentration is 0.1 m.
- b) Reaction was performed on 0.3 mmol scale. Yield in parentheses corresponds to isolated yield.
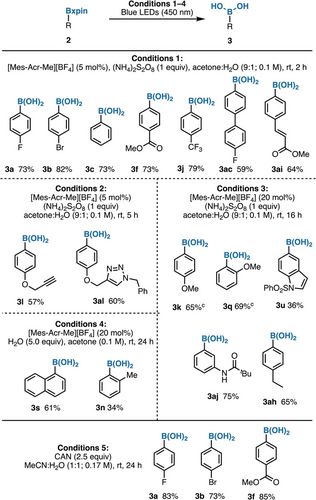
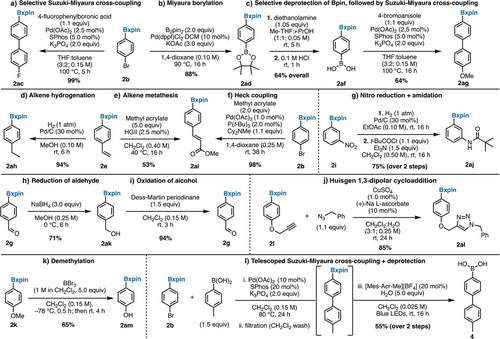
To explore the robustness of xpin boronates as surrogates of boronic acids in orthogonal chemistry, a variety of substituted arylBxpin substrates were subjected to a selection of common synthetic transformations. To begin, Suzuki–Miyaura cross-coupling provided an excellent yield with selective coupling observed only between the organohalide 2b and free boronic acid, without any homocoupling product of 2ab observed (Figure 7a). Additionally, 2b can be converted into a diborylbenzene (2ad) via Miyaura borylation (Figure 7b). The latter can undergo selective Bpin deprotection affording the corresponding boronic acid (2af), which can be subjected to a boronyl-selective Suzuki–Miyaura cross-coupling (Figure 7c). Other transition metal-catalyzed reactions, such as a Pd-catalyzed hydrogenation (Figure 7d), alkene metathesis (Figure 7e), and Heck coupling (Figure 7f), were all amenable to Bxpin-bearing substrates. A 2-step nitro reduction followed by amidation with trimethylacetyl chloride also proved successful (Figure 7g). Aldehyde reduction of 4-formylphenylBxpin (2g) with NaBH4 affords 2ak in good yield (Figure 7h), and the latter can be re-oxidized to 2g without any evidence of B─C bond oxidation (Figure 7i). Remarkably, a Huisgen 1,3-dipolar cycloaddition (Figure 7j) furnished the expected product (2al) in high yield. Furthermore, 4-methoxyphenylBxpin (2k) can be transformed in good yield to the corresponding phenol 2am via BBr3-induced demethylation (Figure 7k). It is noteworthy that some of these transformations fail with the corresponding pinacol esters, which is a testimony to the robustness of xpin boronates. For example, demethylation of 4-MeO-C6H4Bpin with BBr3 instead provides the deborylated phenol (see pages S75 and S76). Lastly, a selective Suzuki–Miyaura coupling was telescoped with a photochemical xpin cleavage without solvent change (Figure 7l).
In summary, xanthopinacol (xpin) boronates are a conceptually new class of trivalent adducts for boronic acids designed to alleviate many of the inconveniences of pinacol esters, such as their labile and reversible nature, and the limitations of robust tetravalent adducts that display limited solubility and require harsh conditions to regenerate the boronic acid. Xpin boronates are cleavable under phororedox catalysis or oxidative conditions that transform the xanthopinacol into xanthone to prevent the re-esterification and facilitate the isolation of the boronic acid. Xpin boronates can be formed directly from xanthone and UV light; they are soluble in a wide range of solvents and tolerate several reaction conditions, allowing for orthogonal chemistry.
Supporting Information
The authors have cited additional references within the Supporting Information.[52-67]
Acknowledgements
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada (Discovery Grant to D.G.H.). The authors thank Dr. Michael J. Ferguson for X-ray crystallographic analysis of 2b.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.