Iridium-Catalyzed Asymmetric β-Selective Hydroamination of Enamides for the Synthesis of 1,2-Diamines
Yu-Wen Sun
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorHao-Tian Tan
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorSheng-Nan Sun
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bi-Jie Li
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Engineering Research Center of Advanced Rare Earth Materials (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]
Search for more papers by this authorYu-Wen Sun
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorHao-Tian Tan
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorSheng-Nan Sun
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Bi-Jie Li
Center of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing, 100084 China
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032 China
Engineering Research Center of Advanced Rare Earth Materials (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing, 100084 China
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
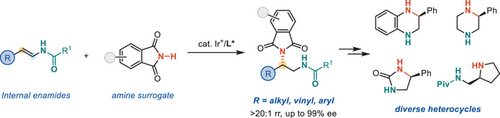
Internal enamides were found to undergo enantioselective hydroamination catalyzed by a cationic iridium complex. An amide group effectively directs the catalyst to achieve unconventional β-regioselectivity with up to 99% ee. This coordination assistance strategy creates an efficient pathway for synthesizing valuable enantioenriched 1,2-diamine derivatives.
Abstract
An iridium-catalyzed highly enantioselective hydroamination of electron-rich alkenes has been developed. The coordination assistance of the amide group to the metal center effectively overrides the inherent electronic preference of N─H addition to an enamide, delivering unconventional β-selectivity. Phthalimide is utilized as a readily removable amination agent. This methodology enables direct access to enantio-enriched 1,2-diamines from readily available materials with 100% atom economy, exclusive regioselectivity, and excellent enantioselectivity (up to 99% ee).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statements
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202507200-supp-0001-SuppMat.pdf8.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 T. C. Nugent, Chiral Amine Synthesis: Methods, Developments and Applications, Wiley-VCH, Weinheim 2010.
10.1002/9783527629541 Google Scholar
- 2S. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451–3479.
- 3J. Mayol-Llinàs, A. Nelson, W. Farnaby, A. Ayscough, Drug Disc. Today 2017, 22, 965–969.
- 4D. Lucet, T. L.e Gall, C. Mioskowski, Angew. Chem. Int. Ed. 1998, 37, 2580–2627.
10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 5S. R. S. Saibabu Kotti, C. Timmons, G. Li, Chem. Biol. Drug Des. 2006, 67, 101–114.
- 6J.-C. Kizirian, Chem. Rev. 2008, 108, 140–205.
- 7D. S. Surry, S. L. Buchwald, Chem. Sci. 2010, 1, 13–31.
- 8T. Ooi, D. Sakai, M. Takeuchi, E. Tayama, K. Maruoka, Angew. Chem. Int. Ed. 2003, 42, 5868–5870.
- 9O. Kitagawa, K. Yotsumoto, M. Kohriyama, Y. Dobashi, T. Taguchi, Org. Lett. 2004, 6, 3605–3607.
- 10M. J. MacDonald, D. J. Schipper, P. J. Ng, J. Moran, A. M. Beauchemin, J. Am. Chem. Soc. 2011, 133, 20100–20103.
- 11E. T. Mwenda, H. N. Nguyen, Org. Lett. 2017, 19, 4814–4817.
- 12D. Perrotta, M. M. Wang, J. Waser, Angew. Chem. Int. Ed. 2018, 57, 5120–5123.
- 13X. Shao, K. Li, S. J. Malcolmson, J. Am. Chem. Soc. 2018, 140, 7083–7087.
- 14Y. Chen, Y. Pan, Y.-M. He, Q.-H. Fan, Angew. Chem. Int. Ed. 2019, 58, 16831–16834.
- 15H.-J. Pan, Y. Lin, T. Gao, K. K. Lau, W. Feng, B. Yang, Y. Zhao, Angew. Chem. Int. Ed. 2021, 60, 18599–18604.
- 16A. Geraci, U. Stojiljković, K. Antien, N. Salameh, O. Baudoin, Angew. Chem. Int. Ed. 2023, 62, e202309263.
- 17M. Zhou, Y. Lin, X.-X. Chen, G. Xu, L. W. Chung, W. Tang, Angew. Chem. Int. Ed. 2023, 62, e202300334.
- 18C. Hervieu, M. S. Kirillova, Y. Hu, S. Cuesta-Galisteo, E. Merino, C. Nevado, Nat. Chem. 2024, 16, 607–614.
- 19S.-C. Wang, L. Liu, M. Duan, W. Xie, J. Han, Y. Xue, Y. Wang, X. Wang, S. Zhu, J. Am. Chem. Soc. 2024, 146, 30626–30636.
- 20R. Bloch, Chem. Rev. 1998, 98, 1407–1438.
- 21K. Manabe, H. Oyamada, K. Sugita, S. Kobayashi, J. Org. Chem. 1999, 64, 8054–8057.
- 22R. Hirabayashi, C. Ogawa, M. Sugiura, S. Kobayashi, J. Am. Chem. Soc. 2001, 123, 9493–9499.
- 23P. Merino, I. Delso, V. Mannucci, T. Tejero, Tetrahedron Lett. 2006, 47, 3311–3314.
- 24D. Uraguchi, N. Kinoshita, T. Kizu, T. Ooi, J. Am. Chem. Soc. 2015, 137, 13768–13771.
- 25S. Izumi, Y. Kobayashi, Y. Takemoto, Org. Lett. 2016, 18, 696–699.
- 26A. Dumoulin, G. Bernadat, G. Masson, J. Org. Chem. 2017, 82, 1775–1789.
- 27Y.-H. Cho, A. Fayol, M. Lautens, Tetrahedron: Asymmetry 2006, 17, 416–427.
- 28Y.-h. Cho, V. Zunic, H. Senboku, M. Olsen, M. Lautens, J. Am. Chem. Soc. 2006, 128, 6837–6846.
- 29K. Arai, S. Lucarini, M. M. Salter, K. Ohta, Y. Yamashita, S. Kobayashi, J. Am. Chem. Soc. 2007, 129, 8103–8111.
- 30B. M. Trost, D. R. Fandrick, T. Brodmann, D. T. Stiles, Angew. Chem. Int. Ed. 2007, 46, 6123–6125.
- 31R. Yu, Y. Yamashita, S. Kobayashi, Adv. Synth. Catal. 2009, 351, 147–152.
- 32B. Wu, J. C. Gallucci, J. R. Parquette, T. V. RajanBabu, Chem. Sci. 2014, 5, 1102–1117.
- 33Z. Chai, P.-J. Yang, H. Zhang, S. Wang, G. Yang, Angew. Chem. Int. Ed. 2017, 56, 650–654.
- 34V. Pozhydaiev, A. Paparesta, J. Moran, D. Lebœuf, Angew. Chem. Int. Ed. 2024, 63, e202411992.
- 35J. Hernández-Toribio, R. G. Arrayás, J. C. Carretero, J. Am. Chem. Soc. 2008, 130, 16150–16151.
- 36S. Kobayashi, R. Yazaki, K. Seki, Y. Yamashita, Angew. Chem. Int. Ed. 2008, 47, 5613–5615.
- 37R. G. Arrayás, J. C. Carretero, Chem. Soc. Rev. 2009, 38, 1940–1948.
- 38B. Ranieri, C. Curti, L. Battistini, A. Sartori, L. Pinna, G. Casiraghi, F. Zanardi, J. Org. Chem. 2011, 76, 10291–10298.
- 39W. Q. Zhang, L. F. Cheng, J. Yu, L. Z. Gong, Angew. Chem. Int. Ed. 2012, 51, 4085–4088.
- 40T. Kano, R. Kobayashi, K. Maruoka, Angew. Chem. Int. Ed. 2015, 54, 8471–8474.
- 41M. Kondo, T. Nishi, T. Hatanaka, Y. Funahashi, S. Nakamura, Angew. Chem. Int. Ed. 2015, 54, 8198–8202.
- 42S. Lin, Y. Kawato, N. Kumagai, M. Shibasaki, Angew. Chem. Int. Ed. 2015, 54, 5183–5186.
- 43D. L. Silverio, P. Fu, E. L. Carswell, M. L. Snapper, A. H. Hoveyda, Tetrahedron Lett. 2015, 56, 3489–3493.
- 44W.-R. Zhu, K. Liu, J. Weng, W.-H. Huang, W.-J. Huang, Q. Chen, N. Lin, G. Lu, Org. Lett. 2020, 22, 5014–5019.
- 45K. i. Yamada, S. J. Harwood, H. Gröger, M. Shibasaki, Angew. Chem. Int. Ed. 1999, 38, 3504–3506.
10.1002/(SICI)1521-3773(19991203)38:23<3504::AID-ANIE3504>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 46K. R. Knudsen, T. Risgaard, N. Nishiwaki, K. V. Gothelf, K. A. Jørgensen, J. Am. Chem. Soc. 2001, 123, 5843–5844.
- 47K. Yamada, G. Moll, M. Shibasaki, Synlett 2001, SI, 980–982.
10.1055/s-2001-14639 Google Scholar
- 48B. M. Nugent, R. A. Yoder, J. N. Johnston, J. Am.Chem. Soc. 2004, 126, 3418–3419.
- 49T. P. Yoon, E. N. Jacobsen, Angew. Chem. Int. Ed. 2005, 44, 466–468.
- 50A. Singh, R. A. Yoder, B. Shen, J. N. Johnston, J. Am. Chem. Soc. 2007, 129, 3466–3467.
- 51B. M. Trost, D. W. Lupton, Org. Lett. 2007, 9, 2023–2026.
- 52A. Singh, J. N. Johnston, J. Am. Chem. Soc. 2008, 130, 5866–5867.
- 53D. Uraguchi, K. Koshimoto, T. Ooi, J. Am. Chem. Soc. 2008, 130, 10878–10879.
- 54T. A. Davis, J. C. Wilt, J. N. Johnston, J. Am. Chem. Soc. 2010, 132, 2880–2882.
- 55S. Handa, V. Gnanadesikan, S. Matsunaga, M. Shibasaki, J. Am. Chem. Soc. 2010, 132, 4925–4934.
- 56D. J. Sprague, A. Singh, J. N. Johnston, Chem. Sci. 2018, 9, 2336–2339.
- 57H. Du, W. Yuan, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 11688–11689.
- 58H. Du, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2008, 130, 8590–8591.
- 59B. Simmons, A. M. Walji, D. W. C. MacMillan, Angew. Chem. Int. Ed. 2009, 48, 4349–4353.
- 60F. C. Sequeira, B. W. Turnpenny, S. R. Chemler, Angew. Chem. Int. Ed. 2010, 49, 6365–6368.
- 61R. G. Cornwall, B. Zhao, Y. Shi, Org. Lett. 2013, 15, 796–799.
- 62E. L. Ingalls, P. A. Sibbald, W. Kaminsky, F. E. Michael, J. Am. Chem. Soc. 2013, 135, 8854–8856.
- 63P. Mizar, A. Laverny, M. El-Sherbini, U. Farid, M. Brown, F. Malmedy, T. Wirth, Chem. Eur. J. 2014, 20, 9910–9913.
- 64B. W. Turnpenny, S. R. Chemler, Chem. Sci. 2014, 5, 1786–1793.
- 65Y. Zhu, R. G. Cornwall, H. Du, B. Zhao, Y. Shi, Acc. Chem. Res. 2014, 47, 3665–3678.
- 66S. Fu, H. Yang, G. Li, Y. Deng, H. Jiang, W. Zeng, Org. Lett. 2015, 17, 1018–1021.
- 67K. Muñiz, L. Barreiro, R. M. Romero, C. Martínez, J. Am. Chem. Soc. 2017, 139, 4354–4357.
- 68F. L. Wang, X. Y. Dong, J. S. Lin, Y. Zeng, G. Y. Jiao, Q. S. Gu, X. Q. Guo, C. L. Ma, X. Y. Liu, Chem 2017, 3, 979–990.
- 69F. Foubelo, C. Nájera, M. G. Retamosa, J. M. Sansano, M. Yus, Chem. Soc. Rev. 2024, 53, 7983–8085.
- 70T. E. Müller, M. Beller, Chem. Rev. 1998, 98, 675–703.
- 71S. Hong, T. J. Marks, Acc. Chem. Res. 2004, 37, 673–686.
- 72K. C. Hultzsch, Adv. Synth. Catal. 2005, 347, 367–391.
- 73T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795–3892.
- 74K. D. Hesp, M. Stradiotto, ChemCatChem 2010, 2, 1192–1207.
- 75L. J. Gooßen, L. Huang, M. Arndt, K. Gooßen, H. Heydt, Chem. Rev. 2015, 115, 2596–2697.
- 76M. Manßen, L. L. Schafer, Chem. Soc. Rev. 2020, 49, 6947–6994.
- 77S. Ma, J. F. Hartwig, Acc. Chem. Res. 2023, 56, 1565–1577.
- 78For representative examples of direct asymmetric N-H additions to strained alkenes, see: D. Milstein, A. L. Casalnuovo, J. C. Calabrese, J. Am. Chem. Soc. 1988, 110, 6738–6744.
- 79R. Dorta, P. Egli, F. Zurcher, A. Togni, J. Am. Chem. Soc. 1997, 119, 10857–10858.
- 80J. Zhou, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 12220–12221.
- 81C. S. Sevov, J. Zhou, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 11960–11963.
- 82H. L. Teng, Y. Luo, B. Wang, L. Zhang, M. Nishiura, Z. Hou, Angew. Chem. Int. Ed. 2016, 55, 15406–15410.
- 83Z. Li, J. Zhao, B. Sun, T. Zhou, M. Liu, S. Liu, M. Zhang, Q. Zhang, J. Am. Chem. Soc. 2017, 139, 11702–11705.
- 84M. Wang, J. C. Simon, M. Xu, S. A. Corio, J. S. Hirschi, V. M. Dong, J. Am. Chem. Soc. 2023, 145, 14573–14580.
- 85A. T. Ho, E. P. Vanable, C. S. Miguel, K. L. Hull, Chem. Commun. 2024, 60, 1615–1618.
- 86For representative examples of direct asymmetric N-H additions to conjugated alkenes, see: M. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2000, 122, 9546–9547.
- 87O. Löber, M. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2001, 123, 4366–4367.
- 88J. Pawlas, Y. Nakao, M. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 3669–3679.
- 89K. L. Butler, M. Tragni, R. A. Widenhoefer, Angew. Chem. Int. Ed. 2012, 51, 5175–5178.
- 90M. L. Cooke, K. Xu, B. Breit, Angew. Chem. Int. Ed. 2012, 51, 10876–10879.
- 91S. Pan, K. Endo, T. Shibata, Org. Lett. 2012, 14, 780–783.
- 92K. Xu, Y. H. Wang, V. Khakyzadeh, B. Breit, Chem. Sci. 2016, 7, 3313–3316.
- 93N. J. Adamson, E. Hull, S. J. Malcolmson, J. Am. Chem. Soc. 2017, 139, 7180–7183.
- 94X. H. Yang, A. Lu, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 14049–14052.
- 95X.-H. Yang, V. M. Dong, J. Am. Chem. Soc. 2017, 139, 1774–1777.
- 96S. Park, S. J. Malcolmson, ACS Catal. 2018, 8, 8468–8476.
- 97G. Tran, W. Shao, C. Mazet, J. Am. Chem. Soc. 2019, 141, 14814–14822.
- 98J. Long, P. Wang, W. Wang, Y. Li, G. Yin, iScience 2019, 22, 369–379.
- 99A. Y. Jiu, H. S. Slocumb, C. S. Yeung, X. H. Yang, V. M. Dong, Angew. Chem. Int. Ed. 2021, 60, 19660–19664.
- 100E. Damer, B. Breit, ChemistryEurope 2024, 2, e202400037.
- 101For representative examples of direct asymmetric N-H additions to unactivated alkenes, see: Z. Zhang, S. D. Lee, R. A. Widenhoefer, J. Am. Chem. Soc. 2009, 131, 5372–5373.
- 102A. L. Reznichenko, H. N. Nguyen, K. C. Hultzsch, Angew. Chem. Int. Ed. 2010, 49, 8984–8987.
- 103C. S. Sevov, J. Zhou, J. F. Hartwig, J. Am. Chem. Soc. 2014, 136, 3200–3207.
- 104S. C. Ensign, E. P. Vanable, G. D. Kortman, L. J. Weir, K. L. Hull, J. Am. Chem. Soc. 2015, 137, 13748–13751.
- 105J. A. Gurak, K. S. Yang, Z. Liu, K. M. Engle, J. Am. Chem. Soc. 2016, 138, 5805–5808.
- 106S. W. Kim, T. Wurm, G. A. Brito, W.-O. Jung, J. R. Zbieg, C. E. Stivala, M. J. Krische, J. Am. Chem. Soc. 2018, 140, 9087–9090.
- 107Y. Xi, S. Ma, J. F. Hartwig, Nature 2020, 588, 254–260.
- 108S. Ma, H. Fan, C. S. Day, Y. Xi, J. F. Hartwig, J. Am. Chem. Soc. 2023, 145, 3875–3881.
- 109S. Ma, Y. Xi, H. Fan, S. Roediger, J. F. Hartwig, Chem 2022, 8, 532–542.
- 110Y.-W. Sun, X. Sun, H.-T. Tan, B.-J. Li, Angew. Chem. Int. Ed. 2025, 64, e202422944.
- 111A. R. Ickes, S. C. Ensign, A. K. Gupta, K. L. Hull, J. Am. Chem. Soc. 2014, 136, 11256–11259.
- 112E. P. Vanable, J. L. Kennemur, L. A. Joyce, R. T. Ruck, D. M. Schultz, K. L. Hull, J. Am. Chem. Soc. 2019, 141, 739–742.
- 113S. Ichikawa, X.-J. Dai, S. L. Buchwald, Org. Lett. 2019, 21, 4370–4373.
- 114Y. Miki, K. Hirano, T. Satoh, M. Miura, Angew. Chem. Int. Ed. 2013, 52, 10830–10834.
- 115J. S. Bandar, M. T. Pirnot, S. L. Buchwald, J. Am. Chem. Soc. 2015, 137, 14812–14818.
- 116Y. Yang, S. L. Shi, D. Niu, P. Liu, S. L. Buchwald, Science 2015, 349, 62–66.
- 117Y. Xi, T. W. Butcher, J. Zhang, J. F. Hartwig, Angew. Chem. Int. Ed. 2016, 55, 776–780.
- 118R. Y. Liu, S. L. Buchwald, Acc. Chem. Res. 2020, 53, 1229–1243.
- 119L. Meng, J. Yang, M. Duan, Y. Wang, S. Zhu, Angew. Chem. Int. Ed. 2021, 60, 23 584–23589.
- 120C. Lee, H. J. Kang, H. Seo, S. Hong, J. Am. Chem. Soc. 2022, 144, 9091–9100.
- 121L. Yu, P. Somfai, Angew. Chem. Int. Ed. 2019, 58, 8551–8555.
- 122M. Mohiti, Y. Lu, H. He, S.-F. Ni, P. Somfai, Chem. Eur. J. 2024, 30, e202303078.
- 123R. Sun, X. Yang, Adv. Synth. Catal. 2024, 366, 4606–4617.
- 124A. M. Johns, N. Sakai, A. Ridder, J. F. Hartwig, J. Am. Chem. Soc. 2006, 128, 9306–9307.
- 125R. A. Hickner, C. I. Judd, W. W. Bakke, J. Org. Chem. 1967, 32, 729–733.
- 126Y. Xie, Y. Zhao, B. Qian, L. Yang, C. Xia, H. Huang, Angew. Chem. Int. Ed. 2011, 50, 5682–5686.
- 127T. Song, Y. Luo, K. Wang, B. Wang, Q. Yuan, W. Zhang, ACS Catal. 2023, 13, 4409–4420.
- 128T. Song, K. Wang, Q. Yuan, W. Zhang, Org. Lett. 2023, 25, 6093–6098.
- 129Y. Du, S. Duan, S. Huang, T. Liu, H. Zhang, P. J. Walsh, X. Yang, J. Am. Chem. Soc. 2024, 146, 30947–30957.
- 130Z. X. Wang, X. Y. Bai, B.-J. Li, Chin. J. Chem. 2019, 37, 1174–1180.
- 131W. Zhao, H.-X. Lu, W.-W. Zhang, B.-J. Li, Acc. Chem. Res. 2023, 56, 308–321.
- 132X.-Y. Bai, Z.-X. Wang, B.-J. Li, Angew. Chem. Int. Ed. 2016, 55, 9007–9011.
- 133X.-Y. Bai, W.-W. Zhang, Q. Li, B.-J. Li, J. Am. Chem. Soc. 2018, 140, 506–514.
- 134S.-L. Zhang, W.-W. Zhang, B.-J. Li, J. Am. Chem. Soc. 2021, 143, 9639–9647.
- 135X.-Y. Bai, W. Zhao, X. Sun, B.-J. Li, J. Am. Chem. Soc. 2019, 141, 19870–19878.
- 136W. Zhao, K.-Z. Chen, A.-Z. Li, B.-J. Li, J. Am. Chem. Soc. 2022, 144, 13071–13078.
- 137For selected reviews, see: A. H. Hoveyda, D. A. Evans, G. C. Fu, Chem. Rev. 1993, 93, 1307–1370.
- 138G. Rousseau, B. Breit, Angew. Chem. Int. Ed. 2011, 50, 2450–2494.
- 139S. Bhadra, H. Yamamoto, Chem. Rev. 2018, 118, 3391–3446.
- 140J. Jeon, C. Lee, I. Park, S. Hong, Chem. Rec. 2021, 21, 3613–3627.
- 141For representative examples, see: S. K. Murphy, A. Bruch, V. M. Dong, Angew. Chem. Int. Ed. 2014, 53, 2455–2459.
- 142R. K. Dhungana, S. KC, P. Basnet, R. Giri, Chem. Rec. 2018, 18, 1314–1340.
- 143G. Wang, X. Liang, L. Chen, Q. Gao, J.-G. Wang, P. Zhang, Q. Peng, S. Xu, Angew. Chem. Int. Ed. 2019, 58, 8187–8191.
- 144D. Qian, S. Bera, X. Hu, J. Am. Chem. Soc. 2021, 143, 1959–1967.
- 145S. Cuesta-Galisteo, J. Schörgenhumer, X. Wei, E. Merino, C. Nevado, Angew. Chem. Int. Ed. 2021, 60, 1605–1609.
- 146F. Zhou, S. Zhu, ACS Catal. 2021, 11, 8766–8773.
- 147Z. Liu, L. J. Oxtoby, M. Liu, Z.-Q. Li, V. T. Tran, Y. Gao, K. M. Engle, J. Am. Chem. Soc. 2021, 143, 8962–8969.
- 148X. Wu, H. Xia, C. Gao, B. Luan, L. Wu, C. Zhang, D. Yang, L. Hou, N. Liu, T. Xia, H. Li, J. Qu, Y. Chen, Nat. Chem. 2024, 16, 398–407.
- 149J.-H. Xie, Q.-L. Zhou, Acc. Chem. Res. 2008, 41, 581–593.
- 150I. P. Beletskaya, A. G. Bessmertnykh, A. D. Averin, F. Denat, R. Guilard, Eur. J. Org. Chem. 2005, 2005, 261–280.
- 151F. T. Ladipo, M. Kooti, J. S. Merola, Inorg. Chem. 1993, 32, 1681–1688.
- 152R. Dorta, A. Togni, Organometallics 1998, 17, 3423–3428.
- 153O. Blum, D. Milstein, J. Am. Chem. Soc. 2002, 124, 11456–11467.
- 154J. I. Seeman, J. Chem. Educ. 1986, 63, 42.