Asymmetric Coordination in Cobalt Single-Atom Catalysts Enables Fast Charge Dynamics and Hierarchical Active Sites for Two-Stage Kinetics in Photodegradation of Organic Pollutants
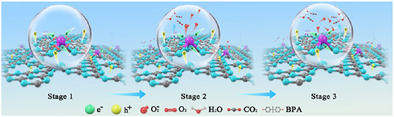
Graphical Abstract
This investigation demonstrates an efficient synthetic methodology for the fabrication of atomically dispersed cobalt species anchored on carbon nitride substrate, featuring distinctive asymmetric Co─C2N3 coordination environments and hierarchical active sites. The unique electronic structure of this single-atom catalyst exhibits strengthened driving force and boosted charge carrier transport properties, manifesting in two-stage reaction kinetics during the photocatalytic degradation of bisphenol A contaminants in wastewater.
Abstract
Single-atom catalysts (SACs) have attracted growing interest in solar-driven catalysis, though challenges persist due to symmetrical metal coordination, which results in limited driving force and sluggish charge dynamics. Additionally, uneven energy and mass distribution complicate reaction pathways, ultimately restricting solar energy utilization and catalytic efficiency. Herein, we synthesized cobalt single atoms decorated carbon nitride catalysts featuring a highly asymmetric Co─C2N3 coordination, tailored for photocatalytic organic pollutants removal. Advanced experimental studies and simulation results collectively revealed that the unique microenvironment surrounding Co single atoms improved charge dynamics and created reactive hot spots, facilitating the generation of reactive oxygen species during the photocatalytic degradation of organic pollutants. These enhanced charge dynamics, combined with hierarchical active sites, resulted in two-stage reaction kinetics and excellent stability for the degradation of bisphenol A in wastewater, distinctly outperforming the first-stage kinetics observed for polymeric carbon nitride. This work advances the understanding of structure-performance relationships in SAC-based photocatalytic degradation and offers valuable insights for the design of next-generation SACs in environmental catalysis.
Introduction
The release of persistent organic pollutants, driven by rapid technological and economic growth, has increasingly impacted daily life, posing significant threats to human health and the environment, with potential long-term effects for future generations.[1, 2] Among these pollutants, bisphenol A (BPA) is particularly hazardous due to its widespread use in daily life, making its effective degradation an urgent environmental priority.[3, 4] Conventional BPA removal methods rely heavily on external energy inputs, such as hydrogen peroxide in Fenton reaction, peroxymonosulfate in Fenton-like processes, and electricity in electrocatalysis.[5-9] In contrast, artificial photodegradation is a promising and sustainable advanced oxidation process, capable of addressing both global energy demands and environmental concerns. Yet, despite nearly five decades of research, the practical application of photocatalysis remains limited by the shortage of affordable and high-efficiency photocatalysts.
Single-atom catalysts (SACs), which feature isolated metal centers dispersed on host matrices, present exciting opportunities for photocatalytic degradation. The architectural configuration of these catalysts, particularly the metal coordination environment, can enhance photon absorption, expedite charge separation and transport, and lower reaction thermodynamic barriers.[10] Modifying the coordination shell of central metal atoms with diverse elements has proven effective in optimizing SAC performance.[11-14] Typically, isolated metal centers are stabilized in symmetric bonding with a single non-metal element, such as four nitrogen atoms (M-N4).[15-17] However, this high symmetry often leads to weak internal electric fields, impeding charge dynamics. To overcome this, breaking the symmetric coordination structure through microenvironment modulation, namely, creating asymmetric coordination structures, can fine-tune the electronic properties of SACs, lowering reaction barriers and improving catalytic efficiency and stability.[18-21] Furthermore, unlike in electrocatalysis and thermocatalysis, SACs in photocatalysis face uneven photon absorption and charge accumulation, and hierarchical active sites, leading to more complex reaction mechanisms. As a result, the fabrication of high-performance SACs with asymmetric coordination structures and understanding their reaction kinetics are urgent in photodegradation for efficient environmental management.
In this work, we proposed an organic ligand restriction strategy to disperse cobalt single atoms on carbon nitride host (CoSA-CN-X, where X refers to the amount of cobalt phytate in precursor). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and X-ray absorption fine structure (XAFS) confirmed the presence of isolated Co atoms in a highly asymmetric Co─C2N3 coordination environment. Experimental and theoretical analyses suggested that this asymmetric coordination modulated the electronic structure of Co centers, thereby facilitating oxygen (O2) adsorption and promoting the generation of key reactive oxygen species (ROS) compared to conventionally symmetric configurations. Moreover, the asymmetric Co─C2N3 coordination accelerated charge dynamics and induced hierarchical active sites, where the metal center accumulated photoexcited electrons and adsorbed O2, while the polymeric carbon nitride (g-C3N4) host gathered photoexcited holes (h+) and adsorbed BPA. In situ characterizations and simulations further demonstrated that ROS generation and consumption occurred at distinct time scales, governed by these hierarchical active sites, leading to two-stage reaction kinetics in BPA photodegradation and significantly boosting ROS production efficiency. Consequently, the optimized CoSA-CN catalyst exhibited excellent BPA degradation performance and remarkable stability, with CoSA-CN-20, maintaining 98.5% degradation efficiency after four cycles under visible light irradiation. Notably, CoSA-CN-20 performed exceptionally well in a flow-type photodegradation unit, achieving a 93.2% pollutant removal rate over 10-h of wastewater treatment. This study underscored the significance of asymmetric coordination structures in modulating the local coordination environments of single-atom centers and provided valuable insights into the structure-activity relationships at the atomic level, particularly in photodegradation kinetics.
Results and Discussion
As shown in Figure 1a, Co-based single-atom photocatalysts were synthesized using a reticular confinement strategy involving the mixing, grinding, and calcination of a mixture of urea and cobalt phytate. Specifically, cobalt phytate was initially prepared from cobalt acetate and phytic acid through a solvothermal approach. CoSA-CN-X was then obtained by directly calcining urea and cobalt phytate without any acid-washing steps. During the polycondensation process, g-C3N4, formed from the polymerization of urea, provided abundant sites for anchoring the metal atoms, while the cobalt phytate ligand served as a confinement agent for the Co centers, preventing agglomeration. This straightforward, clean, and cost−effective synthesis approach enabled the production of single-atom catalysts with varying metal loadings.[23] Inductively coupled plasma-optical emission spectroscopy (ICP-OES) confirmed that Co content in the samples ranged from 0.07% to 1.30% (Table S1).
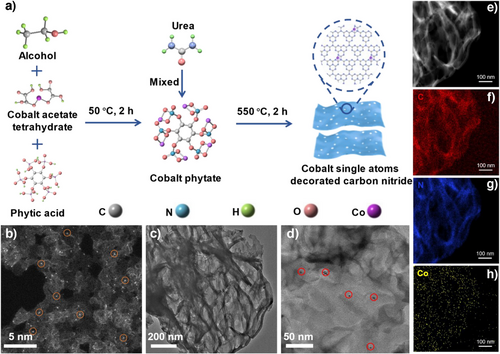
High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image of CoSA-CN-20 (Figure 1b) demonstrated a uniform distribution of Co single atoms across the g-C3N4 framework, with no nanoparticles or clusters detected in the transmission electron microscopy (TEM) image (Figure 1c). In contrast, when cobalt acetate was directly mixed and heated with urea, Co nanoparticles formed on the g-C3N4 substrate (CoNP-CN) (Figure 1d). Energy dispersive spectroscopy-elemental mapping images (Figure 1e–h) of CoSA-CN-20 further confirmed the homogeneous dispersion of isolated Co atoms across the g-C3N4 surface, with no aggregation. The successful anchoring of isolated Co atoms highlights the critical role of cobalt phytate in stabilizing individual metal atoms on the g-C3N4 framework and preventing their aggregation. Additionally, all CoSA-CN samples, regardless of Co loading, exhibited a gauze-like morphology (Figures 1c and S1) similar to those of g-C3N4, indicating that the g-C3N4 framework remained intact during the synthesis process.
Brunauer–Emmett–Teller (BET) analysis was then performed to elucidate the influence of cobalt phytate on the textural structure of g-C3N4 host. The porous nanostructure of CoSA-CN resembled that of g-C3N4, exhibiting a type IV isotherm with H3 hysteresis, indicating a mesoporous structure (Figure S2). The BET surface area of CoSA-CN significantly increased with the increased dosage of cobalt phytate, from 49.4 m2 g−1 of g-C3N4 to 74.2 m2 g−1 of CoSA-CN-20 (Table S2). This increase is attributed to the higher dosage of cobalt phytate in the prepolymer, which produced more gas molecules, like CO2 and H2O, creating larger pores in the g-C3N4 and providing more space to accommodate Co single atoms and reactant adsorption.
The microstructures of the prepared samples were characterized using X-ray diffraction (XRD) patterns, solid-state 13C nuclear magnetic resonance (NMR) spectra, and Fourier transform infrared (FT-IR) spectra. As shown in Figure 2a, XRD patterns of all the samples displayed two diffraction peaks at 2θ values of 27.3° and 13.7°, corresponding to the (002) and (100) planes of g-C3N4, which represent the interlayer stacking and repeated heptazine structural units, respectively.[24] No peaks corresponding to Co nanoparticles were observed, except in the sample of CoNP-CN, which aligns with TEM and HAADF-STEM analyses showing exclusively atomically dispersed Co atoms, without Co clusters or nanoparticles. This atomic dispersion of Co atoms is attributed to their complexation with C and N of g-C3N4 host, facilitating strong metal-support interactions that prevent agglomeration.[23] Besides, the distinctive peaks of g-C3N4 in 13C NMR spectrum (Figure S3a), attributed to the C(1) atom (156.4 ppm) and C(2) atom (164.1 ppm) in the melem unit (Figure S3b), remained unchanged after Co single-atom loading.[25-27] FT-IR spectra (Figure S4) of all CoSA-CN samples also showed absorption bands similar to those of g-C3N4, including CN heterocycles (1200–1700 cm−1) and s-triazine (804 cm−1), confirming that the structural integrity of the heptazine rings was preserved after Co atom incorporation.[28] These results demonstrate that introducing Co single atoms does not alter the chemical structure of the host material, underscoring the efficacy of the synthesis method.
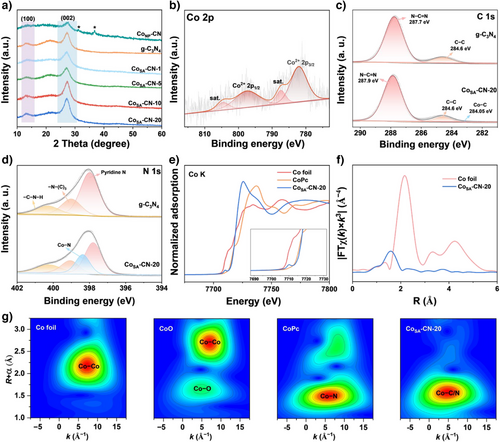
The electronic structures and coordination environments of the prepared samples were further examined using X-ray photoelectron spectroscopy (XPS), synchrotron-based X-ray absorption near-edge structure (XANES), and extended X-ray absorption fine structure (EXAFS). The g-C3N4 material exhibited a N content of 57.2 at% and a C content of 41.4 at% (Table S3), which offers a substantial number of sites for the stabilization of cobalt atoms. In the Co 2p XPS spectrum of CoSA-CN-20 (Figure 2b), the Co 2p3/2 peak at 783.1 eV, along with its satellite peak, confirmed the presence of Co2+ species.[14] In the C 1 s spectra (Figure 2c), g-C3N4 exhibited two peaks at 284.6 and 288.0 eV, corresponding to C─C and N═C─N, respectively.[29] In contrast, an additional peak emerged at 284.1 eV for CoSA-CN-20, which can be assigned to Co─C.[30] In the N 1 s spectra (Figure 2d), characteristic peaks at 398.2, 399.3, and 400.6 eV for g-C3N4 corresponded to pyridinic N, N─(C)3, and C─N─H, respectively.[31, 32] Notably, a new peak at 398.4 eV in CoSA-CN-20 was assigned to Co─N coordination, indicating a metal–N bonding environment.[19]
Synchrotron-based X-ray absorption spectroscopy provided further insights into the atomic structure and local coordination of Co atoms in CoSA-CN-20. As shown in Figure 2e, XANES spectroscopy revealed a pronounced pre-edge peak at 7710 eV for CoSA-CN-20, attributed to the 1 s → 3d transition in Co when coordinated with non-metal elements at the edge sites.[33] The absorption edge position of CoSA-CN-20 was similar to that of cobalt phthalocyanine (CoPc), suggesting a similar valence state (+2) of the Co atom in both materials. Notably, the absorption edge of CoSA-CN-20 shifts to a higher energy compared with CoPc, indicating a lower electron density around Co atoms. This could be attributed to the unique coordination environment of Co single atoms in the g-C3N4 substrate, which might involve both C and N atoms at the same time.[34, 35] The k3-weighted Fourier transform extended X-ray absorption fine structure (FT-EXAFS) spectra at the Co K-edge displayed a single peak at 4.9 Å−1, indicative of Co─N bonds in the first coordination shell, excluding the presence of Co clusters or nanoparticles (Figure 2f). This was further confirmed by wavelet transform EXAFS (WT-EXANES) analysis. The radial distance in the k special resolution of CoSA-CN-20 was 4.8 Å−1 (Figure 2g), similar to CoPc but different from Co foil (about 7.3 Å−1). Furthermore, EXAFS fitting analysis (Table S4) revealed a coordination number of 4.9 for Co with N/C in CoSA-CN-20. To further validate the coordination structure from EXAFS fitting, DFT calculations were performed. Various models (Figure S5) were constructed, and the results revealed that the Co─C2N3 coordination structure possessed the lowest energy, the highest stability, and the lowest chemical binding energy. Integrating these morphological and structural results, it was evident that isolated Co atoms in CoSA-CN-20 preferentially adopt a Co─C2N3 coordination structure, comprising two C atoms and three N atoms.
The photo-absorption properties of the synthesized catalysts significantly affected the photocatalytic performance. Therefore, ultraviolet and visible diffuse reflectance spectra (UV–Vis DRS) were employed to detect the absorption properties of synthesized samples. As shown in Figure 3a, the intrinsic absorption edge of CoSA-CN-X gradually red-shifted with increasing Co single atom loading, indicating enhanced light-trapping capacity. Notably, after the introduction of Co single atoms, Urbach tails appeared in the visible spectral region (circled in Figure 3a), indicative of mid-gap states within the band structure.[36] A progressive increase in the Urbach tail's absorption intensity was observed with higher Co single-atom loading. Consequently, the transition energy (ET) (Figure S6) associated with the Urbach tail was narrowed to 1.92 eV for CoSA-CN-20, enabling the generation of more excitons at lower photon energy. Besides, the band gaps of g-C3N4 and CoSA-CN-20 were estimated to be 2.83 and 2.74 eV, respectively (Figure S7). The narrower band gap of CoSA-CN-20 compared to g-C3N4 is advantageous for solar energy harvesting, promoting better charge carrier separation and transport.[37] XPS analysis further assessed the valence band (VB) positions, with VB edges of 2.07 V for g-C3N4 and 1.82 V for CoSA-CN-20 (Figure S8). Correspondingly, their conduction band (CB) positions were calculated to be −0.76 and −0.92 V, respectively (Figure 3b).
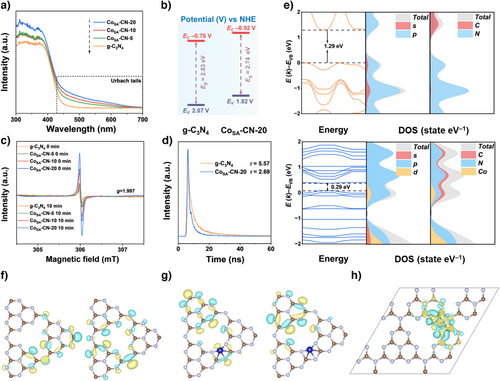
Subsequently, the dynamics of photo-generated charge carriers of the synthesized photocatalysts were thoroughly analyzed using photoluminescence (PL) spectroscopy. As illustrated in Figure S9, a progressive decrease in PL peak intensity was observed with increasing Co atom loading, indicating that the asymmetrical Co─C2N3 sites effectively inhibit the recombination of photo-generated charge carriers, thereby enhancing electron-hole separation. In addition to PL analysis, the excitation of charge carriers within the samples was examined using electron paramagnetic resonance (EPR) measurements, as depicted in Figure 3c. The EPR spectra revealed a single Lorentzian line centered at g values of 1.997 for all catalysts. The quantification of photo-excited hot electrons was achieved by comparing the integrated EPR signal areas under light irradiation and in the dark.[38, 39] A significantly higher EPR signal difference for CoSA-CN-20 than that of g-C3N4 was observed, underscoring the role of asymmetrical Co─C2N3 coordination in promoting charge carrier generation and confirming its positive impact on photocatalytic activity.[40]
Furthermore, time-resolved photoluminescence techniques were used to investigate the lifetimes of photo-induced hot carriers across the samples (Figure 3d). The CoSA-CN-20 sample exhibited a notably shorter average lifetime (τ) compared to g-C3N4, which was attributed to the efficient non-radiative decay pathways facilitated by Co atom incorporation (Table S5).[41] These findings suggested that Co atom anchoring significantly enhances hot carrier mobility within the g-C3N4 matrix. Linear sweep voltammetry (LSV) tests (Figure S10) also demonstrated a lower onset potential for CoSA-CN-20 in the electrocatalytic hydrogen evolution reaction. Complementary Electrochemical Impedance Spectroscopy (EIS) results (Figure S11) revealed a smaller arc radius for CoSA-CN-20, indicating reduced electron transfer resistance and faster charge transport. These results collectively demonstrate that higher Co single-atom loading within the g-C3N4 framework reduces electron transfer resistance and facilitates more efficient charge carrier transport. Consequently, CoSA-CN-20 achieves enhanced separation of photoexcited electrons and h+, significantly improving surface redox reactions. This highlights the critical role of Co atom anchoring in optimizing the photocatalytic performance of g-C3N4-based materials.
Density functional theory (DFT) calculations were further performed to investigate the physiochemical properties of CoSA-CN-20. The interaction between the 3d orbital electrons of Co and the lone pair electrons of pyridinic N, coupled with C-derived 2p states, resulted in the narrowed bandgap of g-C3N4.[42] As shown in the density of states (DOS) results (Figure 3e), the bandgap was significantly reduced from 1.29 eV in pure g-C3N4 to 0.29 eV in CoSA-CN-20. This reduction enhanced photon absorption at lower energies, improving sunlight utilization efficiency across the full spectrum. Highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) results further indicated that, while the electronic distribution in the g-C3N4 model was uniform, the introduction of Co single atoms spatially extended the electronic envelope, which facilitated faster charge separation and transfer (Figure 3f,g). Additionally, the charge density difference (Figure 3h) was calculated to simulate electron transfer behavior. The results revealed that significant electron exchange occurred between the Co doping sites and the adjacent C and N atoms. Clear electron transfer from the non-metallic elements to the Co active sites was observed. These findings, combined with the DOS calculations, confirmed that Co single atoms in SAC improved light absorption and enhanced charge dynamics for photocatalysis.
Based on physicochemical insights, we then evaluated the photocatalytic performance of all synthesized samples in BPA degradation. As shown in Figure 4a, g-C3N4 displayed a degradation rate of only 9.6% under visible light irradiation, while the rate slightly increased to 12% for CoNP-CN due to the presence of nanoparticles. Incorporating Co single atoms into the g-C3N4 host significantly improved degradation performance. The degradation efficiency progressively improved with increasing Co single-atom loading, achieving rates of 34.3%, 51.1%, 81.4%, 98.6%, and 82.3% for CoSA-CN-1, CoSA-CN-5, CoSA-CN-10 CoSA-CN-20, and CoSA-CN-50, respectively. Remarkably, the degradation efficiency decreased when the Co single-atom loading increased from CoSA-CN-20 to CoSA-CN-50 owing to the double-edged effect of metal single atoms, which is consistent with our previous study. [16]
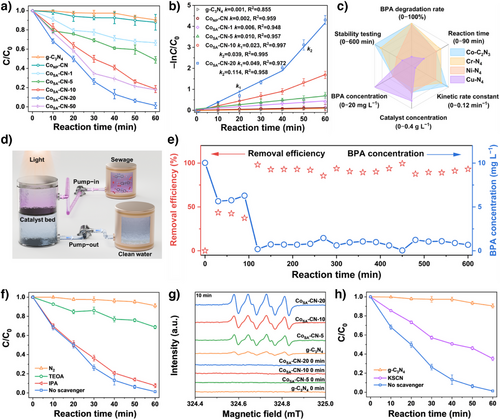
Kinetic plots (−ln(C/C0) versus t) provided a visual comparison of the photocatalytic BPA degradation rates among the synthesized catalysts (Figure 4b). g-C3N4, CoSA-CN-1, CoSA-CN-5, and CoNP-CN systems followed first-order kinetics with rate constants (k) of 0.001, 0.002, 0.006, and 0.010 min−1, respectively. However, a two-stage reaction curve appeared as the Co single atoms loading increased further.[16] The rate constants (k1 and k2) for CoSA-CN-20 were 0.049 and 0.114 min−1, respectively, the highest among all synthetic SACs. Notably, CoSA-CN-20 outperformed both the symmetric M–N4 structured g-C3N4-based catalyst and state-of-the-art photocatalysts reported in previous studies (Figure 4c and Table S6), emphasizing the beneficial role of asymmetric coordination in enhancing photocatalytic performance. Moreover, CoSA-CN-20 maintained high photocatalytic degradation performance over four cycles (Figure S12) and remained highly effective even after 1 year of storage.
To further verify the practical applicability of CoSA-CN-20 in wastewater treatment, it was deposited on a polyvinylidene fluoride (PVDF) membrane and tested in a 10-h flow-type photodegradation process (Figure 4d). As shown in Figure 4e, with 30 mL of wastewater pre-filled in the reactor, CoSA-CN-20 achieved approximately 40% degradation efficiency within the first 1.5 h of continuous operation. The efficiency increased to 98.1% after 2 h and remained stable throughout the 10-h run. XPS and TEM analyses confirmed that the microstructure and metal coordination environment of CoSA-CN-20 remained intact after the reaction, demonstrating its excellent durability and strong potential for practical wastewater treatment applications (Figures S13 and S14).
Quenching experiments were conducted to investigate the ROS involved in the photodegradation process (Figure 4f). Triethanolamine (TEOA, 0.01 M) and isopropanol (IPA, 0.02 M) were chosen as quenchers for h+ and •OH, respectively. The addition of IPA led to a slight reduction in BPA removal efficiency from 98.6% to 92.4%, whereas the introduction of TEOA caused a significant drop in performance from 98.6% to 31.2%. Furthermore, bubbling nitrogen into the solution to remove O2 molecules, thereby inhibiting •O2− generation, reduced the BPA degradation rate to just 8.9%. These results revealed that h+ and •O2− were the primary active species driving BPA photo-oxidation. Besides, EPR experiments using 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) as a spin-trapping reagent confirmed the presence of ROS. Under visible light irradiation, characteristic signals for •O2− (1:1:1:1) were observed, confirming their roles in BPA photo-oxidation, while no signals were detected in the dark (Figure 4g). Quenching either h⁺ or •O2− shifted the BPA degradation kinetics to pseudo-first-order, whereas the secondary-stage kinetics persisted when •OH was quenched (Figure S15), indicating the crucial role of both h⁺ and •O2− in the two-stage photocatalytic degradation of BPA. Besides, potassium thiocyanate (KSCN), which complexes with Co single atoms, was used as a poisoning reagent in BPA degradation experiments to clarify the role of Co single atoms.[45, 46] The degradation rate dropped to 64.8% after KSCN addition (Figure 4h), with reaction kinetics shifting to pseudo-first-order (Figure S16), confirming that Co─C2N3 promoted free radical generation during BPA degradation.
In situ light irradiation XPS was further conducted to explore the hot spots in CoSA-CN-20 driven photodegradation of BPA. Electron binding energy, which correlates with electron density, can provide insights into charge transfer. A binding energy increase reflects electron depletion, while a decrease indicates electron acquisition.[47] As shown in Co 2p spectra (Figure 5a), all peaks under light irradiation shifted downward compared to pre-irradiation, signifying electron accumulation at Co single atoms centers under light exposure.[48] Conversely, upward shifts were noted in the N 1s (Figure 5b) and C 1s (Figure S17) spectra, indicating a reduction in electron cloud density around the C and N atoms. These shifts suggest that photogenerated electrons are transferred from g-C3N4 to Co single atoms under visible light irradiation, leaving photoexcited h+ on g-C3N4 host.

DFT calculations were then performed to unveil the active sites and elucidate the roles of Co─C2N3 in O2 activation and BPA photo-oxidation. Optimized structures of O2/CoSA-CN-20 with different absorption sites representing O2 adsorption on the catalyst surface were displayed in Figure 5c. The adsorption energy of O2 (Eads) on Co single atoms (−0.65 eV) was lower than that on N absorption sites (−0.55 eV), indicating that the anchoring of Co single atoms on the g-C3N4 host could facilitate O2 adsorption. Furthermore, the bond length of the O2 molecule was increased from 1.275 Å on N absorption sites to 1.363 Å at the Co single atoms site (Table S7), suggesting that Co─C2N3 coordination promotes O2 activation into •O2− more effectively. BPA adsorption was also simulated, and the calculated adsorption energies were −0.69 eV on Co single atoms sites and −1.86 eV on N atom absorption sites (Figure 5d). Consequently, BPA was preferentially adsorbed onto the g-C3N4 host and subsequently oxidized by hot h+, which governed the reaction kinetics during the first stage. In the second stage, hot electrons accumulated at the Co─C2N3 sites, facilitating the activation of O2 to generate •O2−. This process, coupled with the action of hot h+, enabled the efficient and rapid removal of BPA.
To gain a comprehensive understanding of BPA degradation within the photocatalytic system, the generated intermediates were analyzed using liquid chromatography–mass spectrometry (LC–MS). Based on the identified oxidation products (Table S8),[49, 50] a detailed pathway for BPA conversion during the photocatalytic degradation system is presented in Figure S18. BPA underwent dehydroxylation and dehydrogenation processes in the photocatalytic reaction, producing intermediates of M1 and M2, respectively. These intermediates then reacted with reactive radicals to form M3, M4 and M5, M6, respectively, through a β-scission (C─C) process.[51, 52] Ultimately, these intermediates underwent ring-opening reactions, leading to their mineralization into CO2 and H2O.
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) provided real-time insight into the adsorption and transformation of BPA by ROS during photocatalytic degradation (Figure 5e). Peaks at 1227, 1310, 1401, 1443, and 1472 cm−1 corresponded to C─N stretching vibrations in the tri-s-triazine heptazine structures of CoSA-CN-20.[53] Subtle shifts in these peaks correlated with the adsorption and desorption processes of BPA and O2 on the catalyst surface. Additional spectral changes further elucidated the degradation pathway. A broad absorption peak centered at 3510 cm−1, assigned to O─H stretching vibrations, was attributed to surface hydroxyl groups, phenolic functionalities of BPA, and adsorbed water.[54] The gradual decrease in its intensity indicated the depletion of hydroxyl groups due to bond cleavage or activation. Moreover, the progressive attenuation and eventual disappearance of the fluctuation peak at 1027 cm−1, corresponding to C─O(H) stretching vibrations, suggested hydroxyl group elimination. The weakening of the aromatic C─H bending vibration at 856 cm−¹ and the C─C stretching vibration at 925 cm−1 further confirmed bond cleavage, highlighting an h+-driven oxidative scission process. These spectral transformations demonstrated a sequential degradation mechanism: initial h+-dominated oxidation followed by radical-coupled degradation pathways.
Additionally, the benzene ring C─H bending vibration peak at 781 cm−1 underwent a redshift under illumination, likely due to the formation of M2, driven by •O2− activation and adsorption on the catalyst surface. Subsequently, the emergence of an upshifted peak at 806 cm−1 indicated further h⁺-mediated activation, triggering structural reorganization of M2 and leading to the formation of M5/M6 intermediates (Figure S18 and Table S8). The appearance of a new peak at 1540 cm−1, assigned to aromatic C═C stretching vibrations, further confirmed the formation of the M6 intermediate. Notably, the C─O stretching vibration peak at 1093 cm−1 initially decreased in intensity before downshifting after 100 min of illumination, illustrating a dual oxidation pathway: direct bond scission by photogenerated h⁺ and oxidative degradation mediated by •O2−.
Based on these findings, the structure-performance relationships and photocatalytic degradation mechanism were proposed. The incorporation of Co single atoms into the g-C3N4 matrix extended the light absorption range, accelerated charge transport, and provided hierarchical active sites for redox reactions, resulting in unique reaction kinetics and superior photodegradation performance. As depicted in Figure 5f, electron-hole pairs were excited within the CoSA-CN-20 photocatalyst under light irradiation (stage 1). Next, the photogenerated h+ on the g-C3N4 substrate contributes to the first-stage kinetics by directly oxidizing BPA. Simultaneously, the hot electrons accumulated at Co─C2N3 sites activated O2 to generate •O2−, which, along with the hot h+, facilitated rapid BPA degradation in the third stage. This two-stage reaction kinetics enhanced the mineralization and degradation of pollutants, achieving a rate constant (k₂) 114 times higher than that of pure g-C3N4. This remarkable performance highlighted the importance of precisely tuning the coordination environment of single-atom centers, optimizing their electronic properties, and ultimately unlocking superior photocatalytic efficiency for sustainable environmental remediation.
Conclusion
In summary, single-atom Co was successfully integrated with a 2D organic semiconductor, g-C3N4, through a simple and environmentally friendly method, and applied for the photodegradation of BPA pollutants. XAFS analysis and DFT calculations revealed that Co atoms anchored in the g-C3N4 framework via an asymmetric Co─C2N3 coordination significantly enhanced solar light absorption and surface electron transfer. Moreover, the charge transfer pathway between the Co single atoms and the surrounding nonmetallic elements was elucidated. The localization of charge electrons around the Co─C2N3 region promoted charge separation, as well as O2 and pollutant adsorption, thereby improving photocatalytic oxidation. Additionally, in situ XPS analysis demonstrated that Co single atoms acted as reactive hot spots, driving the generation of ROS. Complementary in situ DRIFTS and LC–MS results substantiated the presence of a two-stage reaction kinetics mechanism. This study provided new insights into the structure-performance relationship of SACs in photocatalysis and offered valuable guidance for the design of SAC-based photocatalysts for efficient environmental remediation.
Acknowledgements
This work was supported by China Postdoctoral Science Foundation (2023M731363). X.L. acknowledged the China Scholarship Council (CSC) for his Ph.D. grant (202306450070). This work is also partially supported by the Australian Research Council (DP240102707). J.Z. acknowledges the financial support from Australian Research Council via ARC DECRA Fellowship (DE250100753).
Open access publishing facilitated by The University of Adelaide, as part of the Wiley - The University of Adelaide agreement via the Council of Australian University Librarians.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.