Metal-Free Divergent Hydroboration/Multiboration of Terminal Alkynes via Markovnikov Pathway
Dr. Min-Jie Zhou
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
Search for more papers by this authorKe Xu
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
School of Materials Science and Chemical Engineering, Ningbo University, Ningbo, 315211 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yanwei Gu
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Yinjun Xie
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected], [email protected]
Search for more papers by this authorDr. Min-Jie Zhou
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
Search for more papers by this authorKe Xu
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
School of Materials Science and Chemical Engineering, Ningbo University, Ningbo, 315211 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yanwei Gu
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected], [email protected]
Search for more papers by this authorCorresponding Author
Prof. Dr. Yinjun Xie
Zhejiang Key Laboratory of Advanced Fuel Cells and Electrolyzers Technology, Ningbo Institute of Materials Technology and Engineering (NIMTE), Chinese Academy of Sciences, Ningbo, 315201 China
University of Chinese Academy of Sciences, Beijing, 100049 China
E-mail: [email protected], [email protected]
Search for more papers by this authorGraphical Abstract
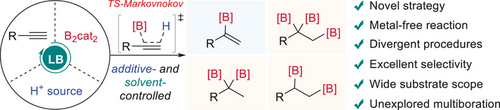
A metal-free and Markovnikov-type process for divergent hydroboration/multiboration of terminal alkynes has been achieved, enabling the transformation of diverse aryl and alkyl alkynes into valuable but previously inaccessible α-alkenylboronate, 2,2-diborylalkane, 1,2,2-triborylalkane, and 1,2-diborylalkane with excellent regio- and chemoselectivities by simply modulating the proton additive and solvent.
Abstract
Hydroboration/multiboration of alkynes has been considered a straightforward route for the construction of high-value alkenylboronates and multiborylalkanes, especially reflected by the productions of β-alkenylboronates and related multiborylalkanes based on the anti-Markovnikov-type transformations of terminal alkyne. However, the syntheses of branched α-alkenylboronates and related multiborylalkanes remain elusive due to the thermodynamically and kinetically unfavorable Markovnikov hydroboration process. Herein, we present a conceptually novel metal-free approach for Markovnikov hydroboration of terminal alkynes to achieve the α-alkenylboronates. Derived from it, we have successfully realized unprecedented tailor-made multiborations (2,2-dihydroboration, 1,2,2-triboration, and 1,2-dihydroboration) of alkynes by simply changing the proton sources and solvents. The broad substrate scope and outstanding chemo- and regioselectivities of the developed approaches unlock opportunities to exploit these formerly unattainable organoboronates, thereby expanding uncharted chemical space. The preliminary mechanistic studies highlight the synergistic roles of amide solvents, suitable proton sources, and B2cat2 in facilitating these tunable transformations.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506968-sup-0001-SupMat.pdf21.4 MB | Supporting Information |
| anie202506968-sup-0002-SupMat.zip360.4 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1D. G. Hall, Medicine and Materials 2nd ed., VCH, Weinheim.
- 2N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483.
- 3S. Namirembe, J. P. Morken, Chem. Soc. Rev. 2019, 48, 3464–3474.
- 4F. Jäkle, Chem. Rev. 2010, 110, 3985–4022.
- 5S. K. Mellerup, S. Wang, Chem. Soc. Rev. 2019, 48, 3537–3549.
- 6S. M. Berger, T. B. Marder, Mater. Horiz. 2022, 9, 112–120, and references therein.
- 7R. J. Grams, W. L. Santos, I. R. Scorei, A. Abad-García, C. A. Rosenblum, A. Bita, H. Cerecetto, C. Viñas, M. A. Soriano-Ursúa, Chem. Rev. 2024, 124, 2441–2511.
- 8I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890–931.
- 9M. Wang, Z. Shi, Chem. Rev. 2020, 120, 7348–7398.
- 10J. Hu, M. Ferger, Z. Shi, T. B. Marder, Chem. Soc. Rev. 2021, 50, 13129–13188.
- 11Y.-M. Tian, X.-N. Guo, H. Braunschweig, U. Radius, T. B. Marder, Chem. Rev. 2021, 121, 3561–3597.
- 12S. K. Bose, L. Mao, L. Kuehn, U. Radius, J. Nekvinda, W. Santos, S. A. Westcott, P. G. Steel, T. B. Marder, Chem. Rev. 2021, 121, 13238–13341.
- 13R. Barbeyron, E. Benedetti, J. Cossy, J.-J. Vasseur, S. Arseniyadis, M. Smietana, Tetrahedron 2014, 70, 8431–8452.
- 14S. Rej, A. Das, T. K. Panda, Adv. Synth. Catal. 2021, 363, 4818–4840.
- 15S. J. Geier, C. M. Vogels, J. A. Melanson, S. A. Westcott, Chem. Soc. Rev. 2022, 51, 8877–8922.
- 16J. Takaya, N. Iwasawa, ACS Catal. 2012, 2, 1993–2006.
- 17R. Nallagonda, K. Padala, A. Masarwa, Org. Biomol. Chem. 2018, 16, 1050–1064.
- 18C. Wu, J. Wang, Tetrahedron Lett. 2018, 59, 2128–2140.
- 19N. Miralles, R. J. Maza, E. Fernández, Adv. Synth. Catal. 2018, 360, 1306–1327.
- 20O. Salvadó, E. Fernández, Molecules 2020, 25, 1758.
- 21X. Wang, Y. Wang, W. Huang, C. Xia, L. Wu, ACS Catal. 2021, 11, 1–18.
- 22C. Gu, D. Gao, J. Org. Chem. 2024, 44, 1385–1402.
- 23K. A. C. Bastick, D. D. Roberts, A. J. B. Watson, Nat. Rev. Chem. 2024, 8, 741–761.
- 24M. Beller, J. Seayad, A. Tillack, H. Jiao, Angew. Chem. Int. Ed. 2004, 43, 3368–3398.
- 25J. Chen, W.-T. Wei, Z. Li, Z. Lu, Chem. Soc. Rev. 2024, 53, 7566–7589.
- 26K. Endo, M. Hirokami, T. Shibata, Synlett 2009, 2009, 1331–1335.
- 27M. Gao, S. B. Thorpe, W. L. Santos, Org. Lett. 2009, 11, 3478–3481.
- 28M. Gao, S. B. Thorpe, C. Kleeberg, C. Slebodnick, T. B. Marder, W. Santos, J. Org. Chem. 2011, 76, 3997–4007.
- 29S. Lee, D. Li, J. Yun, Chem. Asian J. 2014, 9, 2440–2443.
- 30Z. Zuo, Z. Huang, Org. Chem. Front. 2016, 3, 434–438.
- 31G. Gao, Z. Kuang, Q. Song, Org. Chem. Front. 2018, 5, 2249–2253.
- 32J. H. Docherty, K. Nicholson, A. P. Dominey, S. P. Thomas, ACS Catal. 2020, 10, 4686–4691.
- 33H. Kaur, H. Ahuja, R. Arevalo, ACS Catal. 2025, 15, 976–981.
- 34Y. Lee, H. Jang, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 18234–18235.
- 35K. Yang, Q. Song, Green Chem. 2016, 18, 932–936.
- 36G. Gao, J. Yan, K. Yang, F. Chen, Q. Song, Green Chem. 2017, 19, 3997–4001.
- 37K. S. Patil, S. Reddappa, R. Kumar, M. V. Mane, S. K. Bose, J. Org. Chem. 2025, 90, 4140–4148.
- 38X. Yang, S. Ge, Organometallics, 2022, 41, 1823–1828.
- 39P. Nguyen, R. B. Coapes, J. M. Burke, J. A. K. Howard, T. B. Marder, J. Organometal. Chem. 2002, 652, 77–85.
- 40S. Krautwald, M. J. Bezdek, P. J. Chirik, J. Am. Chem. Soc. 2017, 139, 3868–3875.
- 41X. Liu, W. Ming, Y. Zhang, A. Friedrich, T. B. Marder, Angew. Chem. Int. Ed. 2019, 58, 18923–18927.
- 42M. Uzelac, K. Yuan, M. J. Ingleson, Organometallics 2020, 39, 1332–1338.
- 43S. H. Doan, B. K. Mai, T. V. Nguyen, ACS Catal. 2023, 13, 8099–8105.
- 44H. Jang, A. R. Zhugralin, Y. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2011, 133, 7859–7871.
- 45H. Yoshida, Y. Takemoto, K. A. Takaki, Chem. Commun. 2014, 50, 8299–8302.
- 46D. P. Ojha, K. R. Prabhu, Org. Lett. 2016, 18, 432–435.
- 47P. Zhang, J. M. Suárez, T. Driant, E. Driant, Y. Zhang, M. Ménand, S. Roland, M. Sollogoub, Angew. Chem. Int. Ed. 2017, 56, 10821–10825.
- 48C. K. Blasius, V. Vasilenko, R. Matveeva, H. Wadepohl, L. H. Gade, Angew. Chem. Int. Ed. 2020, 59, 23010–23014.
- 49T. Tsushima, H. Tanaka, K. Nakanishi, M. Nakamoto, H. Yoshida, ACS Catal. 2021, 11, 14381–14387.
- 50J. Chen, X. Shen, Z. Lu, Angew. Chem. Int. Ed. 2021, 60, 690–694.
- 51Y. Gao, S. Yazdani, A. Kendrick IV, G. P. Junor, T. Kang, D. B. Grotjahn, G. Bertrand, R. Jazzar, K. M. Engle, Angew. Chem. Int. Ed. 2021, 60, 19871–19878.
- 52P. Wang, I. Douair, Y. Zhao, R. Ge, J. Wang, S. Wang, L. Maron, C. Zhu, Chem 2022, 8, 1361–1375.
- 53A. Martínez-Bascuñana, J. L. Nuñez-Rico, L. Carreras, A. Vidal-Ferran, ACS Catal. 2023, 13, 10447–10456.
- 54T. Tsushima, M. Nakamoto, H. Yoshida, ACS Catal. 2024, 14, 12694–12703.
- 55Y. Wang, Y. Li, L. Wang, S. Ding, L. Song, X. Zhang, Y.-D. Wu, J. Sun, J. Am. Chem. Soc. 2023, 145, 2305–2314.
- 56 Only one example to produce 1,2,2-trialkylboronates have emerged in a patent: X. Wu, L. Zhang, Y. Zhao, R. Chen, Method for synthesizing 1,2,2-triboryl alkane. CN116478195A, Jul 25, 2023.
- 57T. T. Talele, J. Med. Chem. 2020, 63, 13291–13315.
- 58R. D. Dewhurst, E. C. Neeve, H. Braunschweig, T. B. Marder, Chem. Commun. 2015, 51, 9594–9607.
- 59S. Pietsch, E. C. Neeve, D. C. Apperley, R. Bertermann, F. Mo, D. Qiu, M. S. Cheung, L. Dang, J. Wang, U. Radius, Z. Lin, C. Kleeberg, T. B. Marder, Chem. - Eur. J. 2015, 21, 7082–7098.
- 60E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott, T. B. Marder, Chem. Rev. 2016, 116, 9091–9161.
- 61A. B. Cuenca, R. Shishido, H. Ito, E. Fernández, Chem. Soc. Rev. 2017, 46, 415–430.
- 62J. J. Carbó, E. Fernández, Chem. Commun. 2021, 57, 11935–11947.
- 63K. Yang, Q. Song, Acc. Chem. Res. 2021, 54, 2298–2312.
- 64R. Guo, X. Qi, H. Xiang, P. Geaneotes, R. Wang, P. Liu, Y.-M. Wang, Angew. Chem. Int. Ed. 2020, 59, 16651–16660.
- 65P. Nguyen, C. Dai, N. J. Taylor, W. P. Power, T. B. Marder, N. L. Pickett, N. C. Norman, Inorg. Chem. 1995, 34, 4290–4291.
- 66P.-F. Ning, Y. Wei, X.-Y. Chen, Y.-F. Yang, F.-C. Gao, K. Hong, Angew. Chem. Int. Ed. 2024, 63, e202315232.
- 67L. Wang, T. Zhang, W. Sun, Z. He, C. Xia, Y. Lan, C. Liu, J. Am. Chem. Soc. 2017, 139, 5257–5264.
- 68M. Huang, H. Sun, F. Seufert, A. Friedrich, T. B. Marder, J. Hu, Angew. Chem. Int. Ed. 2024, 63, e202401782.
- 69 Deposition numbers 2406182 (for 3g) and 2390656 (for 4h). These data are provided free of charge by the joint Cambridge Crystallographic Data Centre (http://www.ccdc.cam.ac.uk/structures).
- 70N. Miralles, R. Alam, K. J. Szabó, E. Fernández, Angew. Chem. Int. Ed. 2016, 55, 4303–4307.
- 71Y.-X. Chen, F.-C. Gao, P.-F. Ning, Y. Wei, K. Hong, Angew. Chem. Int. Ed. 2023, 62, e202302638.
- 72Z. Kuang, S. Mai, K. Yang, Q. Song, Sci. Bull. 2019, 64, 1685–1690.
- 73R. T. Baker, P. Nguyen, T. B. Marder, S. A. Westcott, Angew. Chem. Int. Ed. 1995, 34, 1336–1338.
- 74A. Viso, R. F. de la Pradilla, M. Tortosa, ACS Catal. 2022, 12, 10603–10620.
- 75J.-H. Zhao, A. Chen, X.-Z. Zou, C.-L. Ji, H.-D. Feng, D.-W. Gao, Chin. J. Chem. 2024, 42, 3484–3498.
- 76Z. Kuang, K. Yang, Y. Zhou, Q. Song, Chem. Commun. 2020, 56, 6469–6479.
- 77N. Xu, Z. Kong, J. Z. Wang, G. J. Lovinger, J. P. Morken, J. Am. Chem. Soc. 2022, 144, 17815–17823.
- 78W. Sun, L. Wang, C. Xia, C. Liu, Angew. Chem. Int. Ed. 2018, 57, 5501–5505.
- 79S. N. Mlynarski, C. H. Schuster, J. P. Morken, Nature 2014, 505, 386–390.
- 80J. Li, H. Wang, Z. Qiu, C.-Y. Huang, C.-J. Li, J. Am. Chem. Soc. 2020, 142, 13011–13020.
- 81W. Clegg, C. Dai, F. J. Lawlor, T. B. Marder, P. Nguyen, N. C. Norman, N. L. Pickett, W. P. Power, A. J. Scott, J. Chem. Soc. Dalton Trans. 1997, 839–846.
- 82V. Fasano, J. Cid, R. J. Procter, E. Ross, M. J. Ingleson, Angew. Chem. 2018, 130, 13477–13481.
- 83J.-F. Ge, X.-Z. Zou, X.-R. Liu, C.-L. Ji, X.-Y. Zhu, D.-W. Gao, Angew. Chem. Int. Ed. 2023, 62, e202307447.
- 84M. Huang, J. Hu, S. Shi, A. Friedrich, J. Krebs, S. A. Westcott, U. Radius, T. B. Marder, Chem. - Eur. J. 2022, 28, e202200480.
- 85A. Bonet, C. Pubill-Ulldemolins, C. Bo, H. Gulyás, E. Fernández, Angew. Chem. Int. Ed. 2011, 50, 7158–7161.