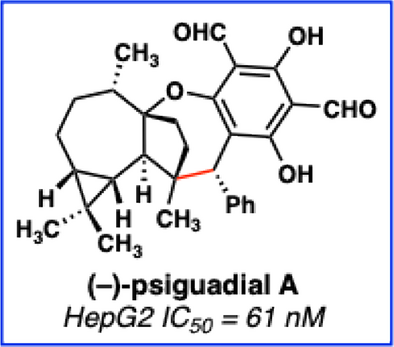
Enantioselective Total Synthesis of (–)-Psiguadial A
Liam P. O'Grady
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMarcel Achtenhagen
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMichael F. Wisthoff
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorRobert S. Lewis
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorKatarina Pfeifer
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorWeifeng Zheng
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMaxwell I. Martin
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorGlenn P. A. Yap
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorCorresponding Author
William J. Chain
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
E-mail: [email protected]
Search for more papers by this authorLiam P. O'Grady
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMarcel Achtenhagen
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMichael F. Wisthoff
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorRobert S. Lewis
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorKatarina Pfeifer
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorWeifeng Zheng
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorMaxwell I. Martin
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorGlenn P. A. Yap
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
Search for more papers by this authorCorresponding Author
William J. Chain
Department of Chemistry & Biochemistry, University of Delaware, 163 The Green, Newark, Delaware, 19716 USA
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
A biomimetic approach harnessing reactive species enabled the enantioselective synthesis of the complex meroterpenoid psiguadial A through an enolate–ortho-quinone methide (oQM) umpolung strategy and late-stage cationic ring closure. The final fully decorated aromatic core was successfully synthesized by adaptation of modern aryl methyl ether deprotection and formylation methodologies, establishing efficient access to this class of natural products.
Abstract
The first enantioselective total synthesis of the antiproliferative natural product (–)-psiguadial A is reported. This approach features the enantioselective synthesis of a complex tricyclic terpenoid precursor, the union of that precursor with a polyketide component by an enolate-ortho-quinone methide coupling reaction to form a highly congested carbon─carbon bond, and an acid-mediated intramolecular hydration ring-closure leveraging a fully substituted alkene to generate the unique oxepane core structure of the natural product.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506537-sup-0001-SuppMat.pdf7.7 MB | Supporting Information |
| anie202506537-sup-0002-SuppMat.cif6.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. H. Tang, Z. Dong, Y. Q. Guo, Z. B. Cheng, C. J. Zhou, S. Yin, Sci. Rep. 2017, 7, 1047.
- 2X. J, Qin, H. Yan, W. Ni, M. Y. Yu, A. Khan, H. Liu, H. X. Zhang, L. He, X. J. Hao, Y. T. Di, H. Y. Liu, Sci. Rep. 2016, 6, 32748.
- 3X. J. Qin, Q. Yu, H. Yan, A. Khan, M. Y. Feng, P. P. Li, X. J. Hao, L. K. An, H. Y. Liu, J. Agric. Food Chem 2017, 65, 4993–4999.
- 4H. Z. Fu, Y. M. Luo, C. J. Li, J. Z. Yang, D. M. Zhang, Org. Lett. 2010, 12, 656–659.
- 5M. Shao, Y. Wang, Z. Liu, D. M. Zhang, H. H. Cao, R. W. Jiang, C. L. Fan, X. Q. Zhang, H. R. Chen, X. S. Yao, W. C. Ye, Org. Lett. 2010, 12, 5040–5043.
- 6I. P. Singh, J. Sidana, S. B. Bharate, W. J. Foley, Nat. Prod. Rep. 2010, 27, 393–416.
- 7M. Nazir, M. Saleem, M. I. Tousif, M. A. Anwar, F. Surup, I. Ali, D. Wang, N. Z. Mamadalieva, E. Alshammari, M. L. Ashour, I. Ahmed, I. R. G. Elizbit, H. Hussain, Biomolecules 2021, 11, 957.
- 8L. M. Chapman, J. C. Beck, L. Wu, S. E. Reisman, J. Am. Chem. Soc. 2016, 138, 9803–9806.
- 9L. M. Chapman, J. C. Beck, C. R. Lacker, L. Wu, S. E. Reisman, J. Org. Chem. 2018, 83, 6066–6085.
- 10A. L. Lawrence, R. M. Adlington, J. E. Baldwin, V. Lee, J. A. Kershaw, A. L. Thompson, Org. Lett. 2010, 12, 1676–1679.
- 11S. B. Bharate, I. P. Singh, Tetrahedron Lett. 2006, 47, 7021–7024.
- 12T. Tanaka, H. Mikamiyama, K. Maeda, C. Iwata, Y. In, T. Ishida, J. Org. Chem. 1998, 63, 9782–9793.
- 13S. Ning, Z. Liu, Z. Wang, M. Liao, Z. Xie, Org. Lett. 2019, 21, 8700–8704.
- 14X. Cheng, N. L. Harzdorf, T. Shaw, D. Siegel, Org. Lett. 2010, 12, 1304–1307.
- 15D. N. Tran, N. Cramer, Chemistry 2014, 20, 10654–10660.
- 16M. Shao, Y. Wang, Y. Q. Jian, X. J. Huang, D. M. Zhang, Q. F. Tang, R. W. Jiang, X. G. Sun, Z. P. Lv, X. Q. Zhang, H. R. Chen, X. S. Yao, W. C. Ye, Org. Lett. 2012, 14, 5262–6265.
- 17R. S. Lewis, C. J. Garza, A. T. Dang, T. K. Pedro, W. J. Chain, Org. Lett. 2015, 17, 2278–2281.
- 18L.-N. Gao, K. Zheng, H.-Y. Chen, Y.-N. Gao, Z.-Z. Li, C. He, S.-H. Huang, R. Hong, M. Bian, Z.-J. Liu, Org. Biomol. Chem. 2025, 23, 2775–2792.
- 19 Deposition numbers 2374950 (for 4), 2446527 (for 4'), 2374947 (for 8), 2374946 (for 15), 2374948 (for 21), 2374949 (for 23), 2374951 (for 24), and 2374945 (for 38) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 20L. Jiao, C. Yu, J. Li, Z. Wang, M. Wu, E. Hao, J. Org. Chem. 2009, 74, 7525–7528.
- 21A. Vilsmeier, A. Haack, A. Ber. 1927, 60, 119–122.
- 22M. J. Classen, M. N. A. Bocker, R. Roth, W. M. Amberg, E. M. Carreira, J. Am. Chem. Soc. 2021, 143, 8261–8265.
- 23R. K. Jackson, 3rd, J. L. Wood, Org. Lett. 2021, 23, 1243–1246.
- 24T. Satoh, Y. Kaneko, T. Okuda, S. Uwaya, K. Yamakawa, Chem. Pharm. Bull. 1984, 32, 3452–3460.
- 25R. L. Funk, J. F. Fitzgerald, T. A. Olmstead, K. S. Para, J. A. Wos, J. Am. Chem. Soc. 1993, 115, 8849–8850.
- 26R. L. Funk, T. A. Olmstead, M. Parvez, J. Am. Chem. Soc. 1988, 110, 3298–3300.
- 27T. Tanaka, Y. Funakoshi, K. Uenaka, K. Maeda, H. Mikamiyama, Y. Takemoto, N. Maezaki, C. Iwata, Chem. Pharm. Bull. 1994, 42, 300–305.
- 28J. Hudon, T. A. Cernak, J. A. Ashenhurst, J. L. Gleason, Angew. Chem. Int. Ed. Engl. 2008, 47, 8885–8888.
- 29C. Dittmer, G. Raabe, L. Hintermann, Eur. J. Org. Chem. 2007, 2007, 5886–5898.
- 30J. L. Hu, M. Bian, H. F. Ding, Tetrahedron Lett. 2016, 57, 5519–5539.
- 31S. Hünig, H. R. Müller, W. Thier, Angew. Chem. Int. Ed. 1965, 4, 271–280 .
- 32C. E. Miller, J. Chem. Educ. 1965, 42, 254–259.
- 33K. Biswas, H. Lin, J. T. Njardarson, M. D. Chappell, T. C. Chou, Y. Guan, W. P. Tong, L. He, S. B. Horwitz, S. J. Danishefsky, J. Am. Chem. Soc. 2002, 124, 9825–9832.
- 34C. W. Wullschleger, J. Gertsch, K. H. Altmann, Org. Lett. 2010, 12, 1120–1123.
- 35A. Suzuki, M. Sasaki, T. Nakagishi, T. Ueda, N. Hoshiya, J. Uenishi, Org. Lett. 2016, 18, 2248–2251.
- 36J. T. Groves, K. W. Ma, J. Am. Chem. Soc. 1977, 99, 4076–4082.
- 37A. A. Torrens, A. L. Ly, D. Fong, A. Adronov, Eur. J. Org. Chem. 2022, e202200570.
- 38A. F. Barrero, E. J. Alvarez-Manzaneda, R. Chahboun, M. Cortés, V. Armstrong, Tetrahedron 1999, 55, 15181–15208.
- 39H. Zhai, L. Zhang, L. Hu, Synlett 2016, 27, 876–879.
- 40A. G. M. Barrett, T. K. Ma, T. Mies, Synthesis 2019, 51, 67–82.
- 41R. S. Kim, L. O. Kgoadi, J. C. Hayes, D. P. Rainboth, C. M. Mudd, G. P. A. Yap, D. A. Watson, J. Am. Chem. Soc. 2024, 146, 17606–17612.
- 42J. Li, C. Zhang, H. Xu, C. Wang, R. Dong, H. Shen, X. Zhuang, X. Chen, Q. Li, J. Lu, M. Zhang, X. Wu, K. M. Loomes, Y. Zhou, Y. Zhang, J. Liu, Y. Xu, J. Med. Chem. 2022, 65, 5760–5799.
- 43S. H. M. Mehr, H. Depmeier, K. Fukuyama, M. Maghami, M. J. MacLachlan, Org. Biomol. Chem. 2017, 15, 581–583.
- 44A. Rieche, H. Gross, E. Höft, Chem. Ber. 1960, 93, 88–94.
- 45G. A. Kraus, J. Mengwasser, W. Maury, C. Oh, Bioorg. Med. Chem. Lett. 2011, 21, 1399–1401.