Dynamic Hydrogen Bonding Tuned Enantioselectivity Control in Cobaloxime-Chiral Amine Cooperative Catalysis
Zhao Liu
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, Hubei, 430072 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Long Zhang
Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Key Laboratory of Organosilicon Material Technology of Zhejiang Province, College of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, 311121 2318 Yuhangtang Road, Hangzhou, P.R. China
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorProf. Sanzhong Luo
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Xiaotian Qi
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, Hubei, 430072 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorZhao Liu
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, Hubei, 430072 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Long Zhang
Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Key Laboratory of Organosilicon Material Technology of Zhejiang Province, College of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, 311121 2318 Yuhangtang Road, Hangzhou, P.R. China
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorProf. Sanzhong Luo
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing, 100084 P.R. China
Search for more papers by this authorCorresponding Author
Prof. Xiaotian Qi
College of Chemistry and Molecular Sciences, State Key Laboratory of Power Grid Environmental Protection, Wuhan University, Wuhan, Hubei, 430072 P.R. China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
Abstract
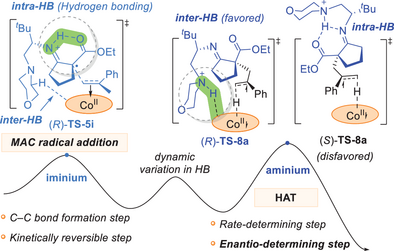
Cobaloxime is a versatile 3d-metal catalyst for dehydrogenative radical coupling reactions. Prior mechanistic studies of cobaloxime catalysis have revealed that cobaloxime(II)-catalyzed hydrogen atom transfer (HAT) process can control the site-selectivity and chemoselectivity of radical transformation. However, the relevance of HAT to the enantioselectivity is seldom reported. Herein, our density functional theory studies of the cobaloxime-chiral amine co-catalyzed enantioselective coupling of the enamine radical cation with alkene proved that it is the HAT step that determines the enantioselectivity, instead of the previously proposed radical addition. Because the metal–alkene-coupled (MAC) radical addition is a reversible process while the followed by HAT is the rate-determining step. Moreover, computational studies suggest that the dynamically changing hydrogen bonding between the radical cation and cobaloxime plays a significant role in harnessing the reactivity and tuning the enantiocontrol. The intra- and inter-molecular hydrogen bonding between the iminium and the cobaloxime fragments contributes to the lower energy barrier of MAC radical addition. Structural analysis and quantitative steric-electronic effect dissection jointly unveiled that the stronger intermolecular hydrogen bonding between the aminium and the cobaloxime fragments is the dominant factor that stabilizes the (R)-HAT transition state and guarantees high enantioselectivity.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202506268-sup-0001-SuppMat.docx57.1 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1K. C. Cartwright, A. M. Davies, J. A. Tunge, Eur. J. Org. Chem. 2020, 2020, 1245–1258.
- 2J. Demarteau, A. Debuigne, C. Detrembleur, Chem. Rev. 2019, 119, 6906–6955.
- 3T. Michiyuki, K. Komeyama, Asian J. Org. Chem. 2020, 9, 343–358.
- 4A. K. Bagdi, M. Rahman, D. Bhattacherjee, G. V. Zyryanov, S. Ghosh, O. N. Chupakhin, A. Hajra, Green Chem. 2020, 22, 6632–6681.
- 5K. Ohmatsu, T. Ooi, Nat. Synth. 2023, 2, 209–216.
- 6B. Chen, L.-Z. Wu, C.-H. Tung, Acc. Chem. Res. 2018, 51, 2512–2523.
- 7M. Kojima, S. Matsunaga, Trend. Chem. 2020, 2, 410–426.
- 8A. Mazzeo, S. Santalla, C. Gaviglio, F. Doctorovich, J. Pellegrino, Inorg. Chim. Acta 2021, 517, 119950.
- 9Z. Ye, Y. M. Lin, L. Gong, Eur. J. Org. Chem. 2021, 2021, 5545–5556.
- 10P. Dam, K. Zuo, L. M. Azofra, O. El-Sepelgy, Angew. Chem. Int. Ed. 2024, 63, e202405775.
- 11J. G. West, D. Huang, E. J. Sorensen, Nat. Commun. 2015, 6, 10093.
- 12P. K. Verma, Coord. Chem. Rev. 2022, 472, 214805.
- 13Y.-B. Kang, P.-F. Dai, Q.-Q. Li, J.-P. Qu, Synthesis 2023, 56, 2213–2222.
- 14L. Koracak, V. Ajdacic, J. Serb. Chem. Soc. 2024, 89, 785–806.
- 15L. Niu, H. Yi, S. Wang, T. Liu, J. Liu, A. Lei, Nat. Commun. 2017, 8, 14226.
- 16H. Zhang, A. Lei, Asian J. Org. Chem. 2018, 7, 1164–1177.
- 17C. Y. Huang, J. Li, C. J. Li, Nat. Commun. 2021, 12, 4010.
- 18J. Li, C.-Y. Huang, C.-J. Li, Trend. Chem. 2022, 4, 479–494.
- 19H. Cao, H. Jiang, H. Feng, J. M. C. Kwan, X. Liu, J. Wu, J. Am. Chem. Soc. 2018, 140, 16360–16367.
- 20X. Hu, G. Zhang, F. Bu, X. Luo, K. Yi, H. Zhang, A. Lei, Chem. Sci. 2018, 9, 1521–1526.
- 21H. Cao, Y. Kuang, X. Shi, K. L. Wong, B. B. Tan, J. M. C. Kwan, X. Liu, J. Wu, Nat. Commun. 2020, 11, 1956.
- 22E. Bergamaschi, C. Weike, V. J. Mayerhofer, I. Funes-Ardoiz, C. J. Teskey, Org. Lett. 2021, 23, 5378–5382.
- 23J. Li, C.-Y. Huang, J.-T. Han, C.-J. Li, ACS Catal. 2021, 11, 14148–14158.
- 24K. C. Cartwright, J. A. Tunge, ACS Catal. 2018, 8, 11801–11806.
- 25G. Zhang, Y. Lin, X. Luo, X. Hu, C. Chen, A. Lei, Nat. Commun. 2018, 9, 1225.
- 26M.-J. Zhou, L. Zhang, G. Liu, C. Xu, Z. Huang, J. Am. Chem. Soc. 2021, 143, 16470–16485.
- 27W.-L. Yu, Z.-G. Ren, K.-X. Ma, H.-Q. Yang, J.-J. Yang, H. Zheng, W. Wu, P.-F. Xu, Chem. Sci. 2022, 13, 7947–7954.
- 28G. Zhang, L. Zhang, H. Yi, Y. Luo, X. Qi, C. H. Tung, L. Z. Wu, A. Lei, Chem. Commun. 2016, 52, 10407–10410.
- 29W. Q. Liu, T. Lei, S. Zhou, X. L. Yang, J. Li, B. Chen, J. Sivaguru, C. H. Tung, L. Z. Wu, J. Am. Chem. Soc. 2019, 141, 13941–13947.
- 30W. L. Yu, Y. C. Luo, L. Yan, D. Liu, Z. Y. Wang, P. F. Xu, Angew. Chem. Int. Ed. 2019, 58, 10941–10945.
- 31S. U. Dighe, F. Julia, A. Luridiana, J. J. Douglas, D. Leonori, Nature 2020, 584, 75–81.
- 32P. G. Lenhert, Chem. Commun. (London) 1967, 3, 980–982.
- 33M. Razavet, V. Artero, M. Fontecave, Inorg. Chem. 2005, 44, 4786–4795.
- 34A. Bakac, M. E. Brynildson, J. H. Espenson, Inorg. Chem. 1986, 25, 4108–4114.
- 35X. Hu, B. M. Cossairt, B. S. Brunschwig, N. S. Lewis, J. C. Peters, Chem. Commun. 2005, 41, 4723.
- 36G. N. Schrauzer, L.-P. Lee, J. W. Sibert, J. Am. Chem. Soc. 1970, 92, 2997–3005.
- 37Y. Nishida, S. Kida, Coord. Chem. Rev. 1979, 27, 275–298.
- 38W. L. M. Baumgarten, Chem. Phys. Lett. 1987, 133, 102–108.
- 39B. E. Daikh, R. G. Finke, J. Am. Chem. Soc. 1992, 114, 2938–2943.
- 40B. P. Branchaud, G. X. Yu, Organometallics 1993, 12, 4262–4264.
- 41H. Lu, W. I. Dzik, X. Xu, L. Wojtas, B. de Bruin, X. P. Zhang, J. Am. Chem. Soc. 2011, 133, 8518–8521.
- 42W. C. C. Lee, X. P. Zhang, Angew. Chem. Int. Ed. 2024, 63, e202320243.
- 43H. B. Gjerde, J. H. Espenson, Organometallics 1982, 1, 435–440.
- 44T. T. Tsou, M. Loots, J. Halpern, J. Am. Chem. Soc. 1982, 104, 623–624.
- 45X.-M. Zhang, J. Org. Chem. 1998, 63, 1872–1877.
- 46S. A. Green, S. W. M. Crossley, J. L. M. Matos, S. Vasquez-Cespedes, S. L. Shevick, R. A. Shenvi, Acc. Chem. Res. 2018, 51, 2628–2640.
- 47L. Capaldo, D. Ravelli, M. Fagnoni, Chem. Rev. 2022, 122, 1875–1924.
- 48D. Zhang, X. Hui, C. Wu, Y. Zhu, ChemCatChem 2021, 13, 3370–3380.
- 49C. Wang, L. M. Azofra, P. Dam, M. Sebek, N. Steinfeldt, J. Rabeah, O. El-Sepelgy, ACS Catal. 2022, 12, 8868–8876.
- 50V. O. Nyagilo, S. C. Mallojjala, J. S. Hirschi, ACS Catal. 2024, 14, 4683–4689.
- 51G. P. Cerai, B. Morandi, Chem. Commun. 2016, 52, 9769–9772.
- 52H. Zhao, A. J. McMillan, T. Constantin, R. C. Mykura, F. Julia, D. Leonori, J. Am. Chem. Soc. 2021, 143, 14806–14813.
- 53S. Wang, Y. Gao, Z. Liu, D. Ren, H. Sun, L. Niu, D. Yang, D. Zhang, X. a. Liang, R. Shi, X. Qi, A. Lei, Nat. Catal. 2022, 5, 642–651.
- 54H. Huang, X. Luan, Z. Zuo, Angew. Chem. Int. Ed. 2024, 63, e202401579.
- 55Y. Wan, E. Ramirez, A. Ford, H. K. Zhang, J. R. Norton, G. Li, J. Am. Chem. Soc. 2024, 146, 4985–4992.
- 56S. Wang, D. Ren, Z. Liu, D. Yang, P. Wang, Y. Gao, X. Qi, A. Lei, Nat. Synth. 2023, 2, 1202–1210.
- 57S. Wang, X. Luo, Y. Wang, Z. Liu, Y. Yu, X. Wang, D. Ren, P. Wang, Y. H. Chen, X. Qi, H. Yi, A. Lei, Nat. Chem. 2024, 16, 1621–1629.
- 58T. D. Beeson, A. Mastracchio, J.-B. Hong, K. Ashton, D. W. C. MacMillan, Science 2007, 316, 582–585.
- 59Y. Qin, L. Zhu, S. Luo, Chem. Rev. 2017, 117, 9433–9520.
- 60X. Xiao, B. X. Shao, Y. J. Lu, Q. Q. Cao, C. N. Xia, F. E. Chen, Adv. Synth. Catal. 2021, 363, 352–387.
- 61N. Chakraborty, B. Das, K. K. Rajbongshi, B. K. Patel, Eur. J. Org. Chem. 2022, 2022, e202200273.
- 62H. Sun, Y. Ma, G. Xiao, D. Kong, Trend Chem. 2024, 6, 684–701.
- 63Q. Yang, L. Zhang, C. Ye, S. Luo, L. Z. Wu, C. H. Tung, Angew. Chem. Int. Ed. 2017, 56, 3694–3698.
- 64Z. Jia, L. Zhang, S. Luo, J. Am. Chem. Soc. 2022, 144, 10705–10710.
- 65M. Cai, L. Zhang, W. Zhang, Q. Lin, S. Luo, Acc. Chem. Res. 2024, 57, 1523–1537.
- 66Z. Jia, L. Cheng, L. Zhang, S. Luo, Nat. Commun. 2024, 15, 4044.
- 67S. Zhang, L. Cheng, J.-Q. Qi, Z. Jia, L. Zhang, L. Jiao, X. Guo, S. Luo, CCS Chemistry 2024, 6, 2420–2426.
- 68P. Pracht, F. Bohle, S. Grimme, Phys. Chem. Chem. Phys. 2020, 22, 7169–7192.
- 69R. G. Parr, L. v. Szentpály, S. Liu, J. Am. Chem. Soc. 1999, 121, 1922–1924.
- 70L. R. Domingo, P. Perez, Org. Biomol. Chem. 2013, 11, 4350.
- 71B. Giese, Angew. Chem. Int. Ed. Engl. 1983, 22, 753–764.
- 72X. Yan, S. Wang, Z. Liu, Y. Luo, P. Wang, W. Shi, X. Qi, Z. Huang, A. Lei, Sci. China Chem. 2022, 65, 762–770.
- 73F. Ye, S. Zheng, Y. Luo, X. Qi, W. Yuan, ACS Catal. 2024, 14, 8505–8517.
- 74X. Pan, C. Fang, M. Fantin, N. Malhotra, W. Y. So, L. A. Peteanu, A. A. Isse, A. Gennaro, P. Liu, K. Matyjaszewski, J. Am. Chem. Soc. 2016, 138, 2411–2425.
- 75W. F. Zheng, J. Chen, X. Qi, Z. Huang, Nat. Chem. 2023, 15, 1672–1682.
- 76F. M. Bickelhaupt, K. N. Houk, Angew. Chem. Int. Ed. 2017, 56, 10070–10086.
- 77S. Liu, Y. Lei, X. Qi, Y. Lan, J. Phys. Chem. A 2014, 118, 2638–2645.
- 78D. H. Ess, K. N. Houk, J. Am. Chem. Soc. 2007, 129, 10646–10647.
- 79T. A. Hamlin, B. J. Levandowski, A. K. Narsaria, K. N. Houk, F. M. Bickelhaupt, Chem. - Eur. J. 2019, 25, 6342–6348.
- 80P. Vermeeren, S. C. C. van der Lubbe, C. F. Guerra, F. M. Bickelhaupt, T. A. Hamlin, Nat. Protoc. 2020, 15, 649–667.
- 81P. Vermeeren, T. A. Hamlin, F. M. Bickelhaupt, Chem. Commun. 2021, 57, 5880–5896.
- 82P. R. Horn, Y. Mao, M. Head-Gordon, J. Chem. Phys. 2016, 144, 114107.
- 83P. R. Horn, Y. Mao, M. Head-Gordon, Phys. Chem. Chem. Phys. 2016, 18, 23067–23079.
- 84X. Qi, D. G. Kohler, K. L. Hull, P. Liu, J. Am. Chem. Soc. 2019, 141, 11892–11904.
- 85M. Xu, Y.-B. Li, H. Wang, F. Glorius, X. Qi, Angew. Chem. Int. Ed. 2025, e202500522.