Lignin Dissolution and Direct Ultrasmall-Lignin-Nanoparticle Formation in Acidic and Alkaline Deep Eutectic Solvents: A Molecular-Level Insight
Graphical Abstract
Structural interpretation and molecular dynamics simulations reveal how molecular moieties and interaction networks influence the π–π stacking structures and conformational states of lignin during dissolution and aggregation: laterally shifted in deep eutectic solvents, offset-stacked in tetrahydrofuran, and stretched in dimethyl sulfoxide.
Abstract
Although deep eutectic solvents (DESs) have demonstrated significant potential in lignin processing, their influence on molecular stacking and conformational evolution during lignin dissolution and nanoparticle formation remains insufficiently understood. Here, we develop a green, straightforward, and single-step approach to produce self-assembled lignin nanoparticles (LNPs). The LNPs obtained using the acidic DES method exhibited a great size reduction, with an average size approximately one-ninth of that produced by conventional solvent-exchange methods. To gain mechanistic insights into the reconstruction, dissolution, and self-assembly of lignin in DESs, we integrate structural characterization with molecular dynamics simulations. Specifically, we simulate the dynamic behavior and configurational states of high-molar-mass lignin models (4,182 g mol−1) in aqueous solvent systems. The results reveal the critical role of molecular structure, intra/intermolecular π–π interactions, stacked conformations, solvent-specific effects in determining the size and compactness of LNPs. Notably, the DES stabilizes lateral-shifted configurations, promoting the formation of small and compact LNPs. In contrast, the tetrahydrofuran/H2O solvent system favors offset-stacked configurations and hydrophobic interactions, leading to larger, spherical LNPs. Overall, our findings offer new insights into the underlying mechanisms of LNP formation using DESs, demonstrating the possibility of regulating and controlling lignin assemblies through solvent parameters.
Introduction
As an alternative approach to conventional biomass fractionation, which prioritizes cellulose valorization, lignin-first biorefinery concepts have recently gained increased attention for the production of lignin fractions with desirable features for various applications.[1, 2] These new concepts, which harness organic solvents, dilute acids, and steam explosion, can isolate more reactive lignin with optimized molar-mass distributions and functionalities.[3-6] However, the lignin structure as well as physical and chemical attributes are strongly linked to the extraction process,[7-9] and the complexities and varieties of the lignin properties could limit its application potential.[10, 11]
Recently, deep eutectic solvents (DESs), i.e., mixtures with eutectic-point temperatures lower than those of their corresponding ideal liquid mixtures,[12] have been intensively explored for biomass treatments to improve cellulose digestibility and hemicellulose hydrolysis, as well as to isolate value-added lignin fractions (Figure S1).[13-15] DESs exhibit numerous fascinating characteristics: some are biodegradable, with low toxicity, and can be formed by simply mixing hydrogen bond acceptors and donors.[9, 16-18] Different DES mixtures have been deployed for lignin fractionation from different bioresources.[19-22] Similar to other lignin-extraction methods, the attributes of the obtained lignin fractions are heavily influenced by the DES formulations and treatment conditions.[23-27]
In addition to the application potential of lignin in its dissolved and macromolecular forms, lignin nanoparticles (LNPs) have gained significant interest in numerous applications, such as tools for drug transport for treating bacterial infections or releasing pesticides, artificial muscle enhancers for producing strong sacrificial bonds, natural adhesives for wood bonding, and novel carbon materials.[28-33] LNPs are conventionally fabricated by solvent-exchange processes involving the assembling of lignin molecules into well-shaped nanoparticles via intramolecular and intermolecular interactions.[29, 34, 35] LNP assemblies and their characteristics, such as their particle sizes and morphologies, are significantly affected by the lignin molecular structure (like hydrophilic and hydrophobic groups) and the deployed solvent system.[28, 36, 37] Studies have shown that solvent polarity, various noncovalent interactions (NCI) (including hydrogen bonding (H-bonding), electrostatic forces, hydrophobic forces, and intrinsic π–π interactions of lignin), as well as nucleation-growth pathways, play essential roles in LNP formation in different solvent systems.[36, 38-40] However, the influence of molecular moieties and interaction networks on π–π stacking geometries and conformational states of lignin during dissolution and aggregation, particularly in DES environments, remains insufficiently understood (Table S1).
In this study, we analyzed the molecular characteristics of lignin fractions isolated in acidic DES comprising lactic acid and choline chloride (ChCl–Lac DES, LCL) and alkaline DES comprising glycerol and potassium carbonate (Gly–K2CO3 DES, KGL) from wheat straw (Table S2). Further, we elaborated the noncovalent interaction networks, conformational evolution, and self-aggregation states of lignin in both DES systems as well as in reference solvents (dimethylsulfoxide (DMSO) and tetrahydrofuran (THF)) via molecular dynamics (MD) simulations. Particularly, the interaction patterns and conformational features of lignin in DESs were analyzed using noncovalent reduced density gradient (RDG) analysis, providing mechanistic insight into the dissolution process. Additionally, the self-assembly process of LNPs in aqueous DESs was investigated by tracking the molecular trajectories of lignin. The root-mean-square deviation (RMSD), average minimum interunit distance as a function of time, ring–ring angle/distance distributions, molecular-density maps, and solvent-accessible surface area (SASA) were analyzed to elucidate the conformational evolution and nanoscale assembly of lignin during the solvent-exchange process.
Results and Discussion
Transformations of the Lignin Molecular Structure During the Acidic and Alkaline DES Treatments
The DES wheat straw lignins, LCL extracted using acidic Lac–ChCl DES and KGL extracted using alkaline Gly–K2CO3 DES, exhibited distinct physicochemical properties. LCL had 38% higher purity but 21% lower yield than KGL (Table S3). However, their monosaccharide contents were only slightly different (Table S3). The thermogravimetric results indicated that KGL degraded at a lower rate than LCL (Table S4). Moreover, LCL exhibited a relatively narrow molar-mass distribution (Figure S2).
Two-dimensional heteronuclear single-quantum correlation nuclear magnetic resonance (2D HSQC NMR) analysis was used to explore the structural properties of the aromatic units as well as the interunit linkages of the DES-isolated lignins. The Lac–ChCl and Gly–K2CO3 DES treatments substantially altered the molecular structure of lignin, as depicted in Figure 1a–d and detailed in the corresponding cross-signal assignments in Table S5. The aliphatic region in the 2D HSQC NMR spectra of LCL (Figure 1a) displayed a characteristic signal at δC/δH 68–72/4.5–5.5, which corresponded to Hibbert's ketones (HK). These ketones, which are typical products of lignin acidolysis, were derived from the arylglycerol–β–aryl ether (β–O–4) substructures.[41-43] Thus, acidic DES triggered simultaneous lignin degradation and dissolution, making it a promising solvent with great potential for industrial-scale lignin fractionation.[16] In contrast, conventional acid hydrolysis of lignin is challenging to scale up due to limited lignin solubility and high process costs.[44, 45]
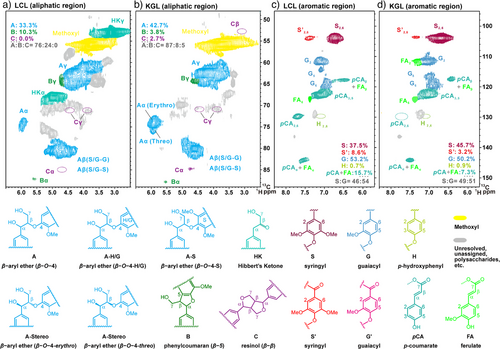
Compared with LCL, KGL displayed a structural characteristic, exhibiting more β–O–4 linkages, including erythro and threo side-chain structures (Figure 1b), as corroborated by its lower phenolic hydroxyl group contents (1.57 mmol g−1 in KGL vs. 1.80 mmol g−1 in LCL, Figure S3). These findings indicate that the mechanism of lignin degradation in alkaline Gly–K2CO3 DES is similar to that of the delignification process of traditional alkaline pulping.[46, 47] However, the alkaline-degradation mechanisms were more complex than the acidolysis ones, and the KGL structure was more similar to that of intact, natural lignin than to that of lignin extracted by alkaline pulping.[48-50] These changes yielded lignin fractions with unique characteristics, impacting their potential subsequent utilization. LCL, with high purity and phenolic hydroxyls (Table S3 and Figure S3), is ideal for bio-based adhesives, phenolic resins, or antioxidants.[2, 51, 52] KGL, with high thermal stability and structural strength, suits applications like composite reinforcement, thermal insulation, or carbon material precursors.[53-56]
Dissolution Mechanisms of Lignin in the Acidic and Alkaline DESs
The molecular-level dissolution mechanisms of lignin in the various DES environments were further elucidated by computational simulations using the guaiacylglycerol–β–guaiacyl ether (GGE) model compound. Particularly, we demonstrated the interrelationship between the structural transformation and dissolution behavior of LCL and KGL. The β–O–4 structure in lignin includes erythro and threo stereostructures, each bearing a pair of enantiomers (Figure 2a). The ratio of these stereostructures varies with the biomass origin.[57] The erythro isomer of GGE was used to simulate the molecular NCIs between lignin and the DES molecules, as it is more abundant in grass lignin, e.g., wheat straw lignin, which was utilized here as our raw material.[58]
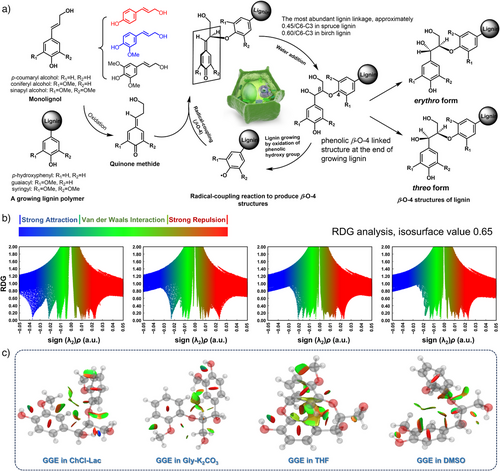
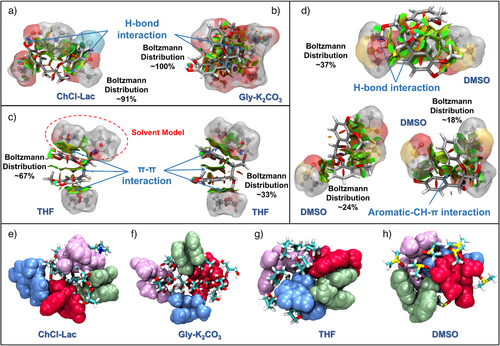
The RDG scatter graph versus electron density multiplied by the sign of the second eigenvalue (sign λ2)ρ was plotted to identify the properties and strengths of the intra/intermolecular NCIs in the whole GGE–solvent molecular systems (Figure 2b). The RDG scatter points presented the corresponding strong attractive interactions (blue region), van der Waals interactions (vdW, green region), and strong repulsive interactions (steric effects, red region). Moreover, the vdW attractions and steric repulsions dominated in the GGE–ChCl–Lac, GGE–Gly–K2CO3, GGE–THF, and GGE–DMSO systems, as shown by the large, color-coded areas. Particularly, the largest scattering was concentrated in the green region, with multiple spikes in the −0.02<sign(λ2)ρ<0.01 range, which is characteristic of strong vdW interactions, as shown in the scatter plots of all the GGE–solvent systems.[59, 60] A spike appeared exclusively in the GGE–ChCl–Lac system around sign(λ2)ρ of −0.04 a.u., indicating strong H-bonding interactions.[61] The isosurface maps corresponding to the NCIs of the most stable GGE configurations under distinct solvent effects are shown in Figure 2c. The disk-shaped color domains indicate NCIs within GGE in the mixed molecular systems. The large green–red domain between the two aromatic rings of the GGE molecule displayed the intramolecular π–π stacking interactions (a strong vdW interaction) in the GGE–THF system, which folded the aromatic rings, generating the offset-stacked GGE configuration. In other GGE–solvent systems, the π–π stacking effect between aromatic rings was relatively weak, and strong repulsive interactions (red domains) existed despite the presence of weak H-bonding interactions (blue–red domains) between the oxygen and hydrogen atoms.
The effects of different solvent environments on the structural conformations of GGE were further illustrated by plotting the NCI isosurfaces of the GGE–solvent systems (Figure 3a–d). The GGE molecule was well solvated in all the investigated solvents via strong intermolecular attractive interactions, as characterized by the large green isosurface domains. The strong H-bonding interactions existed between GGE and the acidic ChCl–Lac or alkaline Gly–K2CO3 DES molecules, as revealed by several small, green disk-like domains, and these interactions reduced the intramolecular stacking. These intra/intermolecular NCIs contributed to the most stable lateral-shifted conformation of GGE presented in the anhydrous ChCl–Lac and Gly–K2CO3 DES systems, accounting for ∼91% and 100% of the Boltzmann distributions, respectively (Figure 3a,b). The GGE–THF system exhibited very strong π–π interactions between two GGE rings, as indicated by the large green–red isosurface disks (Figure 3c), keeping the aromatic rings stacked in an offset mode.[62] Two offset-stacked conformations of GGE with the lowest energy were observed in the THF environment, accounting for 67% and 33% of the Boltzmann distribution. Correspondingly, DMSO induced the stretching of two aromatic rings within GGE. The stretched conformation of GGE with the second lowest energy accounted for more than 24% of the Boltzmann distribution. Additionally, two other stable lateral-shifted conformations were formed (∼37% and 18% of the Boltzmann distribution), mainly exhibiting intramolecular aromatic C─H···π interactions. Thus, GGE dissolution in THF was almost triggered by vdW interactions, and the intramolecular π–π stacking interactions were simultaneously well preserved. In contrast, the DMSO molecule most efficiently disturbed the GGE stacking (Figure 3d). Figure 3e–h further reveals the NCIs of the GGE clusters in four solvent systems (GGE–ChCl–Lac, GGE–Gly–K2CO3, GGE–THF, and GGE–DMSO systems). In the GGE–DES systems, the lateral shifting of the GGE molecules prevented their tight assembly, and the solvent molecules filled the spaces within GGE clusters, partially disrupting their π-stacking interactions. In contrast, in the GGE–THF system, GGE clusters exhibited more compact, offset-stacked configurations, primarily driven by intramolecular π–π interactions, suggesting that THF favored π-stacking compared to DESs. Further, in the DMSO molecular system, intramolecular stacking of GGEs was disrupted, leading to a stretched configuration of GGE clusters.
Formation Mechanisms of Ultrasmall LNPs in Different Solvents
The LNPs were prepared from a direct self-assembling process in aqueous acidic (ChCl–Lac/H2O) and alkaline (Gyl–K2CO3/H2O) DES systems via dialysis; THF and DMSO were employed as reference solvents. The particle size distributions of the LNPs (Figure 4a–f), as determined by the statistical analyses of the field-emission scanning electron microscopy (FESEM) images (Figure 4g–l), indicated the notable variations in the particle sizes of LNPs: 100–180 nm (∼90%) for ChCl–Lac-dialyzed LNPs (LCLNPs), 500–2000 nm (∼82%) for THF-dialyzed LNPs derived from LCL (T_LCLNPs), 40–90 nm (∼82%) for Gly–K2CO3-dialyzed LNPs (KGLNPs), and 200–1000 nm (∼84%) for THF-dialyzed LNPs derived from KGL (T_KGLNPs), respectively. Thus, the average diameters of the particles obtained from acidic and alkaline DES-mediated dialysis (142 nm for LCLNPs and 66 nm for KGLNPs) were approximately one-ninth and one-seventh, respectively, of the diameters of particles obtained from THF-mediated dialysis (1,330 nm for T_LCLNPs and 492 nm for T_KGLNPs). Additionally, the ultrasmall sphere-like nanoparticles formed in DES-dialyzed systems (Figure 4g and j) exhibited a more homogeneous size distribution than that of the spherical LNPs formed in THF-dialyzed systems (Figure 4h and k). When DMSO was exchanged with water molecules during dialysis, it did not trigger lignin aggregation into uniform LNPs (Figure 4i and l). The stability of the LNPs was monitored based on the zeta potential (ζ–potential) of particle dispersions as a function of time (Figure 4m). All the LNPs displayed a high ζ–potential (less than −40 mV) and remained highly stable for 120 days. The ζ–potentials of the DES-obtained LNPs were more negative than those of the THF-dialyzed samples. Additionally, the alkaline DES-dialyzed LNPs exhibited highly negative ζ–potentials, indicating enhanced dispersion stability.
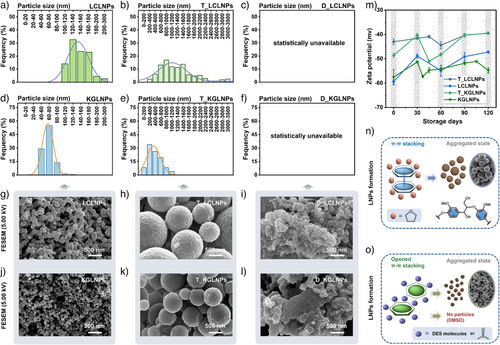
The results indicated that the intra/intermolecular π–π interactions were the key driving forces behind the formation of compact LNPs. Specifically, offset-stacked and lateral-shifted conformations stabilized lignin aggregation, promoting nanoparticle formation. In acidic and alkaline DESs, lignin was strongly solvated through H-bonding and vdW interactions. However, despite this solvation, the lateral-shifting interactions between lignin molecules remained sufficient to drive the formation of nanoparticles. In view of this, the strong weakening of the π–π interactions of lignin induced by the solvent molecules (DMSO) prevented the lignin self-assembling as well as nanoparticle formation during dialysis. These findings highlight the crucial role of solvent properties in determining the formation and characteristics of LNPs (Figures 4n–o). Moreover, DESs used in the dialysis process can be recovered and reused at least five times for the fabrication of LNPs without significant loss of production. In contrast, traditional organic solvents such as THF and DMSO are non-recyclable (Table S6). The yields of DES-LNPs ranged from 96% to 98%, while DES recovery yields were maintained between 90% and 94% over five fabrication cycles (Figure S4). The superior performance of DESs in terms of high dissolution efficiency, excellent solvent recyclability, and overall fabrication sustainability compared to conventional solvents is highlighted in Table S7.
To elucidate the underlying mechanism of the experimental results, we performed MD simulations to investigate the self-assembly behavior of lignin molecules and the formation dynamics of LNPs in the solvent-exchange process. The simulations employed in this study focused on the determination of the structural conformations of a high molar-mass grass-like lignin model (4,182 g mol−1, Figure S5) in different aqueous solvent systems. At a high H2O content (90% by volume), the degree and morphology of the lignin molecules assembled into clusters varied distinctly in different solvent systems (Figures 5a–d and S6). The dynamic trajectories of the lignin molecules over 200 ns are presented in the supplementary animated images. Figure 5e–h show the average number of H-bonds between lignin and other components in the lignin–solvent/H2O mixed systems. In the presence of H2O, the number of H-bonds between lignin and H2O molecules fluctuated between 76 and 116 throughout the simulation, indicating the rapid formation of H-bonding interactions between the lignin and H2O molecules. The number of H-bonds between lignin and H2O decreased in the following sequence: DMSO>ChCl–Lac DES≥Gly–K2CO3 DES>THF. This suggests that the H2O molecules effectively disrupted the existing H-bonds between DESs and lignin, leading to higher lignin dispersion compared to conventional solvent exchange with THF, although still weaker than in DMSO. Further, the number of H-bonds between the lignin and solvent molecules decreased in the following sequence: Gly–K2CO3 DES>ChCl–Lac DES>THF>DMSO, indicating that H-bonding remained in DES/H2O systems after solvent exchange. At equilibrium (after 50 ns), the number of H-bonds between lignin and H2O molecules was much higher than that between the lignin and solvent molecules. This highlights the criticality of H-bonding interactions between H2O and lignin in lignin stacking. This finding is also supported by the number of H-bonds determined in the GGE–solvent/H2O simulation systems (Figures S7a–d).
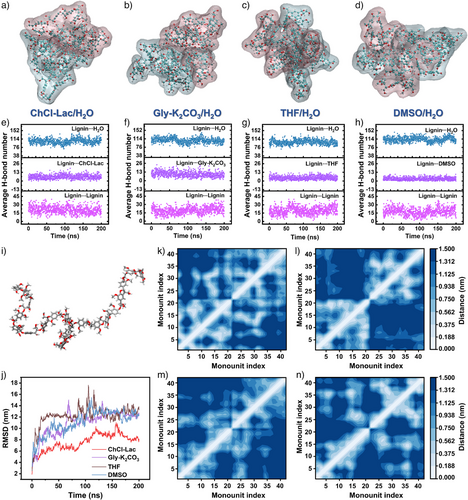
The RMSDs were calculated from 200 ns simulation trajectories to measure the degrees of conformational changes in the lignin clusters at different time steps in relation to the initial conformation (0 ns) (Figure 5j). The fluctuations in the deviation also reflected the stability of the lignin cluster throughout the simulation. The obtained RMSDs of self-assembled lignins increased rapidly with fewer fluctuations, stabilizing at a conformational deviation (13.1Å) after 17 ns in the THF/H2O system. In contrast, the RMSD values in the ChCl–Lac/H2O, Gly–K2CO3/H2O, and DMSO/H2O systems increased gradually with fluctuations, stabilizing after 135 ns, with RMSDs of ∼9.1, 12.7, and 12.9Å, respectively. This indicated that the THF/H2O system was the fastest to reach equilibrium among the other solvent/H2O systems. However, appreciable fluctuations were observed (from 11.5 to 17.6Å) in the 85–120 ns range, indicating significant conformational changes in the lignin clusters in the aqueous THF environment. Large changes were also observed in the lignin configurations in the aqueous Gly–K2CO3 (∼15Å) and DMSO (∼15.5Å) systems. For the ChCl–Lac/H2O system, the RMSD values remained lower than 10Å throughout the 200 ns trajectory, indicating that the configurational structures of the lignin cluster exhibited overall stability. In contrast, THF, DMSO, and the Gly–K2CO3 DES significantly influenced the structural stacking of the lignin molecules in the presence of water (>90 volume%), even though these three systems initially equilibrated before 50ns.
The mean smallest distances between the aromatic units of lignin (Figure 5k–n) were calculated to reflect the specific π–π interactions within the lignin cluster, further characterizing the aggregation tendency of lignin molecules in various solvent/H2O environments. In ChCl–Lac/H2O, Gly–K2CO3/H2O, and THF/H2O environments, most intramolecular aromatic-unit pairs in lignins exhibited distances of less than 0.562 nm, while intermolecular aromatic-unit pairs were spaced at over 1.312 nm (Figure 5k–m). In contrast, in the DMSO/H2O system (Figure 5n), intramolecular aromatic-unit distances of lignin exceeding 1.312 nm had the highest distribution density. Simultaneously, the intermolecular-unit distances of lignin below 0.375 nm were the most frequent (Figure 5n). This indicated the weakening of the intramolecular π–interactions in the presence of DMSO and H2O molecules. Consequently, the lignin molecules aggregated into large clusters, limiting the formation of separate particles. In the ChCl–Lac/H2O (Figure 5k) and Gly–K2CO3/H2O systems (Figure 5l), the average minimum interunit distances within the lignin molecules were predominantly in the 0–0.188 nm, indicating the presence of stronger intramolecular π–interactions. However, the density of the lignin intermolecular-unit pairs with distances of less than 0.188 nm in the ChCl–Lac/H2O system significantly exceeded that in the Gly–K2CO3/H2O (Figure 5l) system, indicating stronger intermolecular π-interactions and leading to the interconnection of some particles during solvent exchange as shown in Figure 4g. Contrarily, the lignin molecules in the THF/H2O environment displayed moderate intra/intermolecular π–stacking interactions with intramolecular-unit distances greater than 0.188 nm and intermolecular-unit distances greater than 0.375 nm (Figure 5m), inducing the formation of larger spherical LNPs as depicted in Figure 4h and k. Thus, the lignin molecules formed relatively small, tightly packed LNPs in the acidic and alkaline DES-mediated dialysis driven by high intramolecular π–π-interaction degrees as well as appropriate intermolecular π–π interactions, which confirmed the stable lateral-shifted conformations of the lignin molecules as shown in Figure 5j. The radical-distribution-function plots of the noncovalent aromatic-carbon pairs within the GGE clusters in the different aqueous-solvent systems revealed similar π–interaction changes (Figures S7e–h).
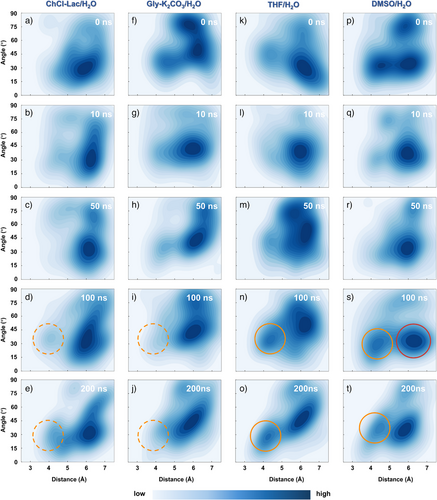
The aromatic ring–ring distances/stacked angles are calculated as a crucial criterion for illustrating the π–stacking interactions (Figures 6 and S8).[63-67] In the deployed ChCl–Lac/H2O and Gly–K2CO3/H2O systems, the π–stacking interactions were observed as indicated by the distances and stacked angles between the aromatic rings, which were distributed in relatively narrow regions of 5–7Å and 15°–60° (Figures 6a–j). As a result, these intra/intermolecular π–stacking interactions promoted the formation of a single dominant laterally shifted conformation in DES/H2O systems. Specifically, throughout the 200 ns simulation, a high-density region was clustered around 6.2Å and 31° in ChCl–Lac/H2O system (Figures 6a–e), while in the Gly–K2CO3/H2O system, the dominant conformation was observed around 6Å and 42°(Figures 6f–j). However, in the THF/H2O and DMSO/H2O systems, more spread-out density distributions were observed within a wide range of 4–7Å and 30°–90°, suggesting that the π-interactions were relatively flexible and multiple stacked configurations emerged (Figures 6k–t). In the 200 ns simulation of the THF/H2O system (Figures 6k–o), the major stacked mode of the aromatic rings with a distance centered on 6Å and a wide range of stacked angles (30°–65°), indicating the diverse conformational distributions with lateral shifting and stretching. Additionally, the aromatic rings of lignin were also densely distributed around 4.3Å and 30° from 100 ns onwards (Figures 6n and o), indicating that the lignin cluster maintained its offset-stacked configurations with strong π-stacking interactions in the equilibrium THF/H2O system.[68] In the DMSO/H2O system, the aromatic interunit stacked angle was concentrated at 30°, and the stacked distances were relatively broad, with two primary distribution centers at 4.5 and 5.8Å, exhibiting offset-stacked and lateral-shifted configurations (Figures 6p–t). Remarkably, within the 100 ns simulation (Figures 6q–s), the stacked aromatic rings were mainly distributed around 6.4Å and 30°, showing stretched conformations, consistent with the results of the GGE model compound simulation (Figure 3d). These results indicate that strong π–stacking interactions in offset-stacked conformations promoted lignin-molecule aggregation into large clusters during THF/H2O and DMSO/H2O exchange (Figure 6n, o, s, and t). In contrast, the absence of offset-stacked configurations of lignin molecules led to the formation of smaller LNPs during DES/H2O exchange, as lateral-shifted conformations dominated (Figure 6d, e, i, and j). Due to the prevalence of stretched configurations, lignin molecules preferentially assembled into plate-like aggregates during DMSO/H2O exchange (Figures 6q–s).
The molecular-density distributions revealed that in acidic (Figure 7a and e) and alkaline (Figure 7b and f) DES/H2O, as well as DMSO/H2O (Figure 7d and h) simulated systems, the trajectories of the solvent molecules were not significantly affected by the lignin molecules, and all the solvent molecules exhibited high miscibility with water. Conversely, the molecular-motion trajectory of THF highly corresponded to that of lignin in the THF/H2O environment (Figure 7c and g). THF molecules mainly accumulate around the lignin cluster, particularly at the trajectories marked by red arrows, at which points lignin–THF complexes form.[69] The results indicated that acidic ChCl–Lac, alkaline Gly–K2CO3, and DMSO were easily removed along with the H2O molecules during solvent exchange, whereas the THF molecules were relatively difficult to remove due to their stronger interaction with lignin.
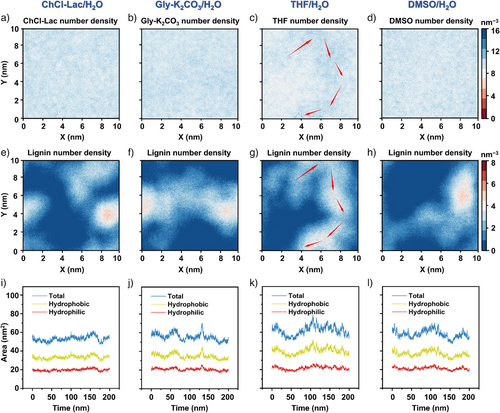
Further analysis of the SASA plots over 200 ns of MD simulation revealed the dynamic behavior and aggregation tendencies of lignins in different aqueous solvent systems (Figures 7i–l). Overall, the total SASA values (blue line) varied across the four mixed solvent systems. THF/H2O (Figure 7k) and DMSO/H2O (Figure 7l) exhibited higher total SASA values (∼61.89 and ∼58.04 nm2, respectively) than DES/H2O systems (∼53.12 nm2 in ChCl–Lac/H2O (Figure 7i) and ∼55.70 nm2 in Gly–K2CO3/H2O (Figure 7j)). This suggests that DES/H2O exhibited weaker solvation effects, promoting the formation of smaller LNP.
In all systems, the hydrophilic SASA values (red line) were around 20.0 nm2 (Figure 7i–l), suggesting weak hydrophilic interactions between lignin and solvent molecules in the presence of water (90% volume). In contrast, the hydrophobic SASA values (yellow line) observed in the THF/H2O (∼40.21 nm2) and DMSO/H2O (∼36.8 nm2) systems were larger than those in the Gly–K2CO3/H2O (∼33.3 nm2) and ChCl–Lac/H2O (∼34.9 nm2) systems, indicating stronger hydrophobic interactions in aqueous THF and DMSO environments, which facilitated larger cluster formation.
Dynamic fluctuations in total SASA values further supported these observed trends. The ChCl–Lac/H2O system (Figure 7i) showed the slightest fluctuations (between 46.6 and 62.4 nm2), indicating stable solvation conformations of lignin over 200 ns simulation. In contrast, the total SASA values in the THF/H2O system (Figure 7k) showed the most significant fluctuations (between 50.8 and 78.5 nm2), indicating frequent conformational changes. Gly–K2CO3/H2O (46.4–70.4 nm2) and DMSO/H2O (47.3–69.9 nm2) showed intermediate variations (Figure 7j and l).
These results suggest that the addition of water rapidly weakens the interactions between DES molecules and both the hydrophilic and hydrophobic regions of lignin. Consequently, this weakening of interactions inhibits the extensive aggregation of lignin molecules, resulting in the formation of smaller LNPs during solvent exchange. In contrast, stronger hydrophobic interactions in THF/H2O and DMSO/H2O promote larger clusters.
Conclusions
In this study, we explored the effects of acidic ChCl–Lac and alkaline Gly–K2CO3 DESs on the molecular reconstruction, dissolution mechanisms, and assembly behavior of lignin through experiments and MD simulations. 2D HSQC NMR analyses revealed that the ChCl–Lac DES cleaved the Cβ–O bonds in the β–O–4 linkages, generating HKs and increasing phenolic hydroxyl groups. In contrast, the Gly–K2CO3 DES induced a lower degree of depolymerization, leading to the preservation of higher content of β–O–4 linkages. NCI computations demonstrated that the intra/intermolecular vdW interactions and π–π interactions accounted for the primary forces governing the molecular solubilization and conformational reorganization of lignin in the solvents, resulting in the following stable solvated lignin conformations: laterally shifted in DES, offset-stacked in THF, and stretched in DMSO. In DES/H2O systems, the lignin molecules adopted a single dominant laterally shifted conformation with reduced hydrophobic interactions, leading to the formation of ultrasmall-packed LNPs. In contrast, THF/H2O favored offset stacking and increased the hydrophobic surface exposure of lignin molecules, promoting the formation of larger spherical LNPs. Meanwhile, the prevalence of stretched configurations during DMSO/H2O exchange led lignin molecules to preferentially assemble into plate-like aggregates. Overall, this study provides molecular-level insights into how solvent environments influence the configurational evolution and assembly behavior of lignin, offering a foundation for future advancements in lignin-based nanomaterials.
Supporting Information
The authors have cited additional references within the Supporting Information.[70-93]
Acknowledgements
This study was supported by the BioKainuu project (VN/24008/2021-OKM-1), the National Natural Science Foundation of China (No. 22208061), the Walter Ahlström Foundation (No. 20240271), the European Research Council (No. 772110), and the Research Council of Finland (No. 340099, 355001). Authors gratefully acknowledge the support from KAUTE foundation, Finnish Cultural Foundation Kalle and Dagmar Välimaan Fund, Kvantum institute (University of Oulu), and the Centre for Material Analysis (University of Oulu).
Open access publishing facilitated by Oulun yliopisto, as part of the Wiley - FinELib agreement.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.





