Quaternary Phosphonium Salts Enable Palladium-Catalyzed Annulative C─H Activation of Aminophosphines with Alkynes
Lin Lin
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorTianbao Wu
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Minyan Wang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorDr. Ronghui Huang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorProf. Dr. Xiuxiu Yang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorDr. Yue Zhao
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhuangzhi Shi
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, 210023 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorLin Lin
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorTianbao Wu
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Minyan Wang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorDr. Ronghui Huang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorProf. Dr. Xiuxiu Yang
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorDr. Yue Zhao
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Zhuangzhi Shi
State Key Laboratory of Coordination Chemistry, Chemistry and Biomedicine Innovation Center (ChemBIC), School of Chemistry and Chemical Engineering, Nanjing University, Nanjing, 210093 China
School of Chemistry and Materials Science, Nanjing Normal University, Nanjing, 210023 China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan, 453007 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
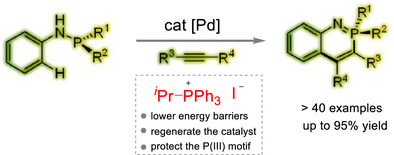
We developed a palladium-catalyzed system for P(III)-directed C─H activation of aminophosphines and cyclization with alkynes facilitated by deprotonative isomerization, using phosphonium salts as key additives. These salts lower energy barriers, regenerate the catalyst, and protect the P(III) group, enabling stereospecific synthesis of chiral phosphacycles.
Abstract
Quaternary phosphonium salts, despite their extensive historical study, have remained unexplored as key additives in transition metal catalysis until our current investigation. Here, we present a groundbreaking palladium-catalyzed system for P(III)-directed C─H activation of aminophosphines, followed by annulation with alkynes. By utilizing P-stereogenic aminophosphines, we have achieved the stereospecific synthesis of chiral phosphacycles. This innovative process, facilitated by deprotonative isomerization, efficiently yields a wide range of P-heterocycles. The pivotal breakthrough of our methodology lies in the identification of phosphonium salts as effective additives, which undertake multiple functions: they not only reduce the energy barrier of pivotal steps but also regenerate the palladium catalyst while safeguarding the P(III) directing group from oxidation. Through systematic experimental investigations, particularly by comparing transition states with and without quaternary phosphonium salts, we have elucidated the unique reaction pathway, thereby explaining the critical role of the salts.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202505454-sup-0001-SuppMat.pdf13.1 MB | Supporting Information |
| anie202505454-sup-0002-SuppMat.zip530.2 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. Fernández-Pérez, P. Etayo, A. Panossian, A. Vidal-Ferran, Chem. Rev. 2011, 111, 2119–2176.
- 2A. Nordheider, J. D. Woollins, T. Chivers, Chem. Rev. 2015, 115, 10378–10406.
- 3B. Roy, A. Depaix, C. Périgaud, S. Peyrottes, Chem. Rev. 2016, 116, 7854–7897.
- 4H. Guo, Y. C. Fan, Z. Sun, Y. Wu, O. Kwon, Chem. Rev. 2018, 118, 10049–10293.
- 5J. B. Rodriguez, C. Gallo-Rodriguez, ChemMedChem 2019, 14, 190–216.
- 6M. Hirai, N. Tanaka, M. Sakai, S. Yamaguchi, Chem. Rev. 2019, 119, 8291–8331.
- 7T. Imamoto, Chem. Rev. 2024, 124, 8657–8739.
- 8P. Seweryn, L. B. Van, M. Kjeldgaard, C. J. Russo, L. A. Passmore, B. Hove-Jensen, B. Jochimsen, D. E. Brodersen, Nature 2015, 525, 68–72.
- 9D. A. DiRocco, Y. Ji, E. C. Sherer, A. Klapars, M. Reibarkh, J. Dropinski, R. Mathew, P. Maligres, A. M. Hyde, J. Limanto, A. Brunskill, R. T. Ruck, L.-C. Campeau, I. W. Davies, Science 2017, 356, 426–430.
- 10K. Wu, A. G. Doyle, Nat. Chem. 2017, 9, 779–784.
- 11A. L. Featherston, Y. Kwon, M. M. Pompeo, O. D. Engl, D. K. Leahy, S. J. Miller, Science 2021, 371, 702–707.
- 12D. L. Scott, J. Cammarata, M. Schimpf, R. Wolf, Nat. Chem. 2021, 13, 458–464.
- 13B. Wang, L. Qin, T. Mu, Z. Xue, G. Gao, Chem. Rev. 2017, 117, 7113–7131.
- 14F.-A. Kang, Z. Sui, W. V. Murray, J. Am. Chem. Soc. 2008, 130, 11300–11302.
- 15E. V. Jennings, K. Nikitin, Y. Ortin, D. G. Gilheany, J. Am. Chem. Soc. 2014, 136, 16217–16226.
- 16G.-Y. Ran, X.-X. Yang, J.-F. Yue, W. Du, Y.-C. Chen, Angew. Chem. Int. Ed. 2019, 58, 9210–9214; Angew. Chem. 2019, 131, 9308–9312.
- 17H. Cheng, X. Wang, L. Chang, Y. Chen, L. Chu, Z. Zuo, Sci. Bull. 2019, 64, 1896–1901.
- 18S. Fang, J.-P. Tan, J. Pan, H. Zhang, Y. Chen, X. Ren, T. Wang, Angew. Chem. Int. Ed. 2021, 60, 14921–14930; Angew. Chem. 2021, 133, 15048–15057.
- 19S. Lu, T. Zheng, J. Ma, Z. Deng, S. Qin, Y. Chen, Y. Liang, Angew. Chem. Int. Ed. 2022, 61, e202201285; Angew. Chem. 2022, 134, e202201285.
- 20J.-H. Wu, J.-P. Tan, J.-Y. Zheng, J. He, Z. Song, Z. Su, T. Wang, Angew. Chem. Int. Ed. 2023, 62, e202215720; Angew. Chem. 2023, 135, e202215720.
- 21L. Pang, Z. Huang, Q. Sun, G. Li, J. Liu, B. Li, C. Ma, J. Guo, C. Yao, J. Yu, Q. Li, Nat. Commun. 2023, 14, 4437–4447.
- 22B. E. Maryanoff, A. B. Reitz, Chem. Rev. 1989, 89, 863–927.
- 23D. Uraguchi, S. Sakaki, T. Ooi, J. Am. Chem. Soc. 2007, 129, 12392–12393.
- 24R. He, X. Wang, T. Hashimoto, K. Maruoka, Angew. Chem. Int. Ed. 2008, 47, 9466–9468; Angew. Chem. 2008, 120, 9608–9610.
- 25M. C. Hilton, X. Zhang, B. T. Boyle, J. V. Alegre-Requena, R. S. Paton, A. McNally, Science 2018, 362, 799–804.
- 26J. L. Koniarczyk, J. W. Greenwood, J. V. Alegre-Requena, R. S. Paton, A. McNally, Angew. Chem. Int. Ed. 2019, 58, 14882–14886; Angew. Chem. 2019, 131, 15024–15028.
- 27J. N. Levy, J. V. Alegre-Requena, R. Liu, R. S. Paton, A. McNally, J. Am. Chem. Soc. 2020, 142, 11295–11305.
- 28X. Zhang, K. G. Nottingham, C. Patel, J. V. A.-R. J. N. Levy, J. N. Levy, R. S. Paton, A. McNally, Nature 2021, 594, 217–222.
- 29S. Roediger, E. L. Saux, P. Boehm, B. Morandi, Nature 2024, 636, 108–114.
- 30B. H. R. Suryanto, K. Matuszek, J. Choi 1, R. Y. Hodgetts, H.-L. Du, J. M. Bakker, C. S. M. Kang, P. V. Cherepanov, A. N. Simonov, D. R. MacFarlane, Science 2021, 372, 1187–1191.
- 31R. G. Bergman, Nature 2007, 446, 391–393.
- 32J. Wencel-Delord, T. Dröge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740.
- 33Z. Dong, Z. Ren, S. J. Thompson, Y. Xu, G. Dong, Chem. Rev. 2017, 117, 9333–9403.
- 34Y. Yang, J. Lan, J. You, Chem. Rev. 2017, 117, 8787–8863.
- 35C.-S. Wang, P. H. Dixneuf, J.-F. Soulé, Chem. Rev. 2018, 118, 7532–7585.
- 36H. M. L. Davies, K. Liao, Nat. Rev. Chem. 2019, 3, 347–360.
- 37P. Gandeepan, T. Müller, D. Zell, G. Cera, S. Warratz, L. Ackermann, Chem. Rev. 2019, 119, 2192–2452.
- 38L. Guillemard, N. Kaplaneris, L. Ackermann, M. J. Johansson, Nat. Rev. Chem. 2021, 5, 522–545.
- 39B. Zhao, B. Prabagar, Z. Shi, Chem 2021, 7, 2585–2634.
- 40T. Dalton, T. Faber, F. Glorius, ACS Cent. Sci. 2021, 7, 245–261.
- 41T. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147–1169.
- 42D. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624–655.
- 43F. Zhang, D. R. Spring, Chem. Soc. Rev. 2014, 43, 6906–6919.
- 44C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603–6743.
- 45B. Liu, A. M. Romine, C. Z. Rubel, K. M. Engle, B.-F. Shi, Chem. Rev. 2021, 121, 14957–15074.
- 46Z. Li, Z. Shi, Acc. Chem. Res. 2024, 57, 1057–1072.
- 47X. Qiu, M. Wang, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2017, 56, 7233–7237; Angew. Chem. 2017, 129, 7339–7343.
- 48D. Wang, B. Dong, Y. Wang, J. Qian, J. Zhu, Y. Zhao, Z. Shi, Nat. Commun. 2019, 10, 3539–3548.
- 49D. Wang, Y. Zhao, C. Yuan, J. Wen, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2019, 58, 12529–12533; Angew. Chem. 2019, 131, 12659–12663.
- 50J. Wen, D. Wang, J. Qian, D. Wang, C. Zhu, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2019, 58, 2078–2082; Angew. Chem. 2019, 131, 2100–2104.
- 51J. Wen, B. Dong, J. Zhu, Y. Zhao, Z. Shi, Angew. Chem., Int. Ed. 2020, 59, 10909–10912; Angew. Chem. 2020, 132, 11001–11004.
- 52D. Wang, M. Li, C. Shuang, Y. Liang, Y. Zhao, M. Wang, Z. Shi, Nat. Commun. 2022, 13, 2934–2943.
- 53Z. Li, M. Wang, Y. Yang, Y. Liang, X. Chen, Y. Zhao, K. N. Houk, Z. Shi, Nat. Commun. 2023, 14, 8509–8518.
- 54W. Jiang, X. Yang, L. Lin, C. Yan, Y. Zhao, M. Wang, Z. Shi, Angew. Chem. Int. Ed. 2023, 62, e202309709; Angew. Chem. 2023, 135, e202309709.
- 55L. Lin, X.-j. Zhang, X. Xu, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2023, 62, e202214584; Angew. Chem. 2023, 135, e202214584.
- 56X. Lv, M. Wang, Y. Zhao, Z. Shi, J. Am. Chem. Soc. 2024, 146, 3483–3491.
- 57Z. Li, W. Xu, S. Song, M. Wang, Y. Zhao, Z. Shi, Angew. Chem. Int. Ed. 2024, 63, e202316035; Angew. Chem. 2024, 136, e202316035.
- 58K. M. Crawford, T. R. Ramseyer, C. J. A. Daley, T. B. Clark, Angew. Chem. Int. Ed. 2014, 53, 7589–7593; Angew. Chem. 2014, 126, 7719–7723.
- 59J.-F. Yang, R.-H. Wang, Y.-X. Wang, W.-W. Yao, Q.-S. Liu, M. Ye, Angew. Chem. Int. Ed. 2016, 55, 14116–14120; Angew. Chem. 2016, 128, 14322–14326.
- 60X. Luo, J. Yuan, C.-D. Yue, Z.-Y. Zhang, J. Chen, G.-A. Yu, C.-M. Che, Org. Lett. 2018, 20, 1810–1814.
- 61K. Fukuda, N. Iwasawa, J. Takaya, Angew. Chem. Int. Ed. 2019, 58, 2850–2853; Angew. Chem. 2019, 131, 2876–2879.
- 62J.-W. Li, L.-N. Wang, M. Li, P.-T. Tang, X.-P. Luo, M. Kurmoo, Y.-J. Liu, M.-H. Zeng, Org. Lett. 2019, 21, 2885–2889.
- 63Z. Zhang, T. Roisnel, P. H. Dixneuf, J.-F. Soulé, Angew. Chem. Int. Ed. 2019, 58, 14110–14114; Angew. Chem. 2019, 131, 14248–14252.
- 64J.-W. Li, L.-N. Wang, M. Li, P.-T. Tang, N.-J. Zhang, T. Li, X.-P. Luo, M. Kurmoo, Y.-J. Liu, M.-H. Zeng, Org. Lett. 2020, 22, 1331–1335.
- 65Z. Zhang, M. Cordier, P. H. Dixneuf, J.-F. Soulé, Org. Lett. 2020, 22, 5936–5940.
- 66W.-T. Ma, M.-G. Huang, Y. Fu, Z.-H. Wang, J.-Y. Tao, J.-W. Li, Y.-J. Liu, M.-H. Zeng, Chem. Commun. 2022, 58, 7152–7155.
- 67J. Rzayev, Z. Zhang, N. Durand, J.-F. Soulé, Org. Lett. 2022, 24, 6755–6760.
- 68Z. Yu, Q. Liu, Y. Yang, J. You, ACS Catal. 2022, 12, 11743–11748.
- 69Z. Zhang, P. H. Dixneuf, J.-F. Soulé, Chem. Commun. 2018, 54, 7265–7280.
- 70J. Zhang, L. Yao, J.-Y. Su, Y.-Z. Liu, Q. Wang, W. P. Deng, Green Synth. Catal. 2023, 4, 206–225.
- 71J. Wen, Z. Shi, Acc. Chem. Res. 2021, 54, 1723–1736.
- 72X. Qiu, H. Deng, Y. Zhao, Z. Shi, Sci. Adv. 2018, 4, eaau6468.
- 73A. J. Borah, Z. Shi, J. Am. Chem. Soc. 2018, 140, 6062–6066.
- 74X. Han, Y. Yuan, Z. Shi, J. Org. Chem. 2019, 84, 12764–12772.
- 75X. Qiu, P. Wang, D. Wang, M. Wang, Y. Yuan, Z. Shi, Angew. Chem. Int. Ed. 2019, 58, 1504–1508; Angew. Chem. 2019, 131, 1518–1522.
- 76B. Dong, J. Qian, M. Li, Z.-J. Wang, M. Wang, D. Wang, C. Yuan, Y. Han, Y. Zhao, Z. Shi, Sci. Adv. 2020, 6, eabd1378.
- 77D. Wang, M. Li, X. Chen, M. Wang, Y. Liang, Y. Zhao, K. N. Houk, Z. Shi, Angew. Chem. Int. Ed. 2021, 60, 7066–7071; Angew. Chem. 2021, 133, 7142–7147.
- 78C. Jacob, J. Annibaletto, J. Peng, R. Bai, B. U. W. Maes, Y. Lan, G. Evano, Angew. Chem. Int. Ed. 2024, 63, e202403553; Angew. Chem. 2024, 136, e202403553.
- 79S. D. Venkataramu, G. D. MacDonell, W. R. Purdum, M. El-Deek, K. D. Berlin, Chem. Rev. 1977, 77, 121–181.
- 80L. Nyulászi, Chem. Rev. 2001, 101, 1229–1246.
- 81R. K. Bansal, J. Heinicke, Chem. Rev. 2001, 101, 3549–3578.
- 82M. J. S. Dewar, V. P. Kubba, J. Am. Chem. Soc. 1960, 82, 5685–5688.
- 83C. Bedel, A. Foucault, Tetrahedron Lett. 1991, 32, 2619–2620.
- 84A. Foucault, C. Bedel, Tetrahedron 1995, 51, 9625–9632.
10.1016/0040-4020(95)00537-I Google Scholar
- 85P. N. M. Botman, O. David, A. Amore, J. Dinkelaar, M. T. Vlaar, K. Goubitz, J. Fraanje, H. Schenk, H. Hiemstra, J. H. van Maarseveen, Angew. Chem. Int. Ed. 2004, 43, 3471–3473; Angew. Chem. 2004, 116, 3553–3555.
- 86H. Deng, M. Wang, Y. Liang, X. Chen, T. Wang, J. J. Wong, Y. Zhao, K. N. Houk, Z. Shi, Chem 2022, 8, 569–579.
- 87R. Huang, M. Wang, H. Deng, J. Xu, H. Yan, Y. Zhao, Z. Shi, Sci. Adv. 2023, 9, eade8638.
- 88T. León, M. Parera, A. Roglans, A. Riera, X. Verdaguer, Angew. Chem. Int. Ed. 2012, 51, 6951–6955; Angew. Chem. 2012, 124, 7057–7061.
- 89See Supporting Information for details. Deposition numbers 2371149, 2371150, 2371151, 2371152, 2371153, 2371154, 2371155, 2371156, 2371157, and 2447353 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge by the Cambridge Crystallographic Data Centre.
- 90H. J. Bestmann, Angew. Chem. Int. Ed. 1965, 4, 830–838.
- 91L. Zong, Y. Gong, Y. Yu, Y. Xie, G. Xie, Q. Peng, Q. Li, Z. Li, Sci. Bull. 2018, 63, 108–116.
- 92B. Han, D. Li, X. Lei, Q. Liu, Y. Chen, Q. Yan, J. Wang, G. He, Chem. Eng. J. 2022, 450, 137933.
- 93Q. Zhou, X. Zhang, L. Ning, Y. Song, Y. Wang, J. Feng, C.-L. Sun, J. Li, Q. Gong, Q. Zhang, Y. Huang, Small Methods 2025, 9, 2401439.
- 94R. Hua, A. Qina, B. Z. Tang, Prog. Polym. Sci. 2020, 100, 101176.
10.1016/j.progpolymsci.2019.101176 Google Scholar
- 95C. Liu, X. Wang, J. Liu, Q. Yue, S. Chen, J. W. Y. Lam, L. Luo, B. Z. Tang, Adv. Mater. 2020, 32, 2004685.
- 96Z. Li, B. Z. Tang, D. Wang, Adv. Mater. 2024, 36, 2406047.
- 97S. Kamino, Y. Horio, S. Komeda, K. Minoura, H. Ichikawa, J. Horigome, A. Tatsumi, S. Kaji, T. Yamaguchi, Y. Usami, S. Hirota, S. Enomotoab, Y. Fujita, Chem. Commun. 2010, 46, 9013–9015.
- 98W. Zhu, L. Huang, C. Wu, L. Liu, H. Li, Luminescence 2024, 39, e4655.
- 99Q. Zhao, J. Z. Sun, J. Mater. Chem. C 2016, 4, 10588–10609.
- 100S. J. Young, B. Kellenberger, J. H. Reibenspies, S. E. Himmel, M. Manning, O. P. Anderson, J. K. Stille, J. Am. Chem. Soc. 1988, 110, 5744–5753.
- 101K. L. Hull, M. S. Sanford, J. Am. Chem. Soc. 2009, 131, 9651–9653.
- 102D. P. Hruszkewycz, J. Wu, N. Hazari, C. D. Incarvito, J. Am. Chem. Soc. 2011, 133, 3280–3283.
- 103B. E. Haines, J. F. Berry, J.-Q. Yu, D. G. Musaev, ACS Catal. 2016, 6, 829–839.
- 104N. Sivendran, N. Pirkl, Z. Hu, A. Doppiu, L. J. Gooßen, Angew. Chem. Int. Ed. 2021, 60, 25151–25160; Angew. Chem. 2021, 133, 25355–25364.
- 105D. L. Davies, S. M. Donald, S. A. Macgregor, J. Am. Chem. Soc. 2005, 127, 13754–13755.
- 106D. García-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2006, 128, 1066–1067.
- 107D. García-Cuadrado, P. de Mendoza, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2007, 129, 6880–6886.
- 108S. I. Gorelsky, D. Lapointe, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 10848–10849.
- 109D. Lapointe, K. Fagnou, Chem. Lett. 2010, 39, 1118–1126.
- 110L. Ackermann, Chem. Rev. 2011, 111, 1315–1345.
- 111D. L. Davies, S. A. Macgregor, C. L. McMullin, Chem. Rev. 2017, 117, 8649–8709.
- 112L. Wang, B. P. Carrow, ACS Catal. 2019, 9, 6821–6836.
- 113All calculations were performed at the M06/6-311+G(d,p)-SDD/SMD(N,N-dimethylformamide)//M06/6-31G(d)-SDD level of theory using Gaussian 09 software package (see Supporting Information for details).