Tetranuclear Titanium Nitride Cluster: Dinitrogen Cleavage Under Mild Conditions and Its Reactivity
Graphical Abstract
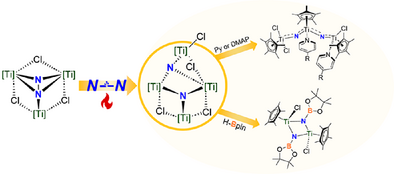
Synopsis: Heating the trinuclear Ti dinitrogen complex [{TiCp*(µ-Cl)}3(µ3-η1: η2: η2-N2)] 1(Cp* = η5-C5Me5) leads to the cleavage of N≡N bond, forming a tetranuclear titanium nitride cluster [{TiCp*}4Cl(µ-Cl)3(µ3-N)2] 2. Complex 2 reacts with pinacolborane, leading to the formation of N-B bond derivatives [{TiCp*(µ-NBpin)Cl}2]. When treated with pyridine or DMAP, complex 2 was dissociated to form a bent trinuclear Ti nitrido complex.
Abstract
Heating the readily available trinuclear Ti dinitrogen complex [{TiCp*(µ-Cl)}3(µ3-η1: η2: η2-N2)] 1(Cp* = η5-C5Me5), originally reported by C. Yélamos and J. Jover, in benzene at 80 °C leads to the cleavage of N≡N bond, forming a tetranuclear titanium nitride cluster [{TiCp*}4Cl(µ-Cl)3(µ3-N)2] 2. The structure of 2 was confirmed by single-crystal X-ray diffraction analysis. Kinetics study and density functional theory (DFT) calculations indicate that the energy barrier for the NN bond cleavage in the transformation from 1 to 2 is about 26 kcal mol−1. Complex 2 exhibits reactivity toward various electrophiles, producing a range of nitrogen-containing compounds in the presence of air, while concurrently regenerating Ti(IV) salts. Furthermore, complex 2 reacts with pinacolborane, leading to the formation of N-B bond derivatives [{TiCp*(µ-NBPin)Cl}2] 3. When complex 2–15N is treated with pyridine or 4-dimethylaminopyridine (DMAP), it dissociates to form a bent trinuclear Ti nitrido complex [{TiCp*}3Cl3(L)2(µ-15N)2] 4-L (L = py or DMAP).
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article. Deposition numbers CCDC 2364905 (2), 2364908 (3), 2412515 (4-py), 2412519 (4-DMAP), 2364907 (5), and 2375502 (6) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and FIZ Karlsruhe Access Structures service.