Deconstructive Functionalization of Cyclic Ketones via Electrochemical Interrupted Dowd–Beckwith Reaction
Graphical Abstract
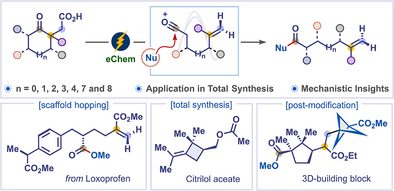
Interrupted Dowd–Beckwith (IDB) reaction enables the highly regioselective deconstructive functionalization of cyclic ketones using electricity as the sole oxidant. This strategy transforms complex cyclic ketones into acyclic molecules and has been applied to the asymmetric synthesis of planococcol, citrilol acetate, maconelliol, and its derivatives. Mechanistic studies support an oxidative radical-polar crossover.
Abstract
Deconstructive functionalization of cyclic molecules has recently emerged as a prominent strategy to access unique architectures that are challenging to prepare through traditional methods. While significant progress has been made in the deconstructive functionalization of cyclic alcohols and amines, the strategies for deconstructing cyclic ketones remain largely unexplored. The Dowd–Beckwith reaction, a ring-expansion of cyclic ketones, is a powerful method for synthesizing medium- and large-ring compounds. Herein, we developed the first interrupted Dowd–Beckwith (IDB) reaction for highly regioselective deconstructive functionalization of cyclic ketones using electricity as the sole oxidant. This protocol is widely applicable for the deconstruction of small, medium-sized, and macrocyclic ketones bearing a diverse range of functional groups. Remarkably, various naturally occurring complex cyclic ketones were successfully deconstructed into acyclic molecules, which are difficult to access by known strategies. The method was applied to the asymmetric synthesis of planococcol, citrilol acetate, maconelliol, and its derivatives. Furthermore, the functional groups incorporated during the transformation provided versatile handles for subsequent synthetic modifications. Mechanistic experiments and computational studies support an oxidative radical-polar crossover followed by deconstructive functionalization.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.