Mechanistic Insights into Cation Effects in Electrolytes for Electrocatalysis
Qian Wu
School of Material Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
Search for more papers by this authorCorresponding Author
Zhichuan J. Xu
School of Material Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
Center for Advanced Catalysis Science and Technology, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
E-mail: [email protected]
Search for more papers by this authorQian Wu
School of Material Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
Search for more papers by this authorCorresponding Author
Zhichuan J. Xu
School of Material Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
Center for Advanced Catalysis Science and Technology, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798 Singapore
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
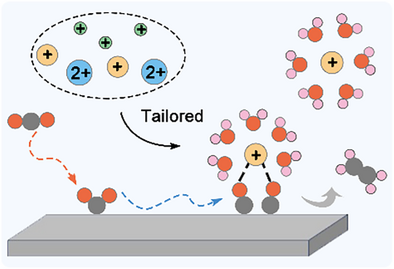
Cations play a pivotal role in controlling electrocatalytic reaction pathways, selectivity, and kinetics. A deeper mechanistic understanding of cation effects offers opportunities to overcome the activity–selectivity trade-off in electrocatalytic reactions, enabling the rational design of more efficient electrolyte systems.
Abstract
Traditional understanding of electrocatalytic reactions has primarily focused on the covalent interactions between adsorbates and catalyst surfaces, often overlooking the significant influence of the electrolyte on the catalytic process. Recently, researchers have highlighted the pivotal role of cations in the electrolyte, demonstrating their capability to modulate the reaction pathways and thus the activity and selectivity. These findings underscore the need for a deeper, atomic-level understanding of the electrode–electrolyte interface. This perspective presents the mechanisms through which cations affect electrocatalysis, with a focus on the hydrogen-bond network, the adsorption behavior of reaction intermediates, the electric field within the electrochemical double layer, and the local electronic properties of catalysts. It provides a summary of the influences of cations on various electrocatalytic reactions, including hydrogen oxidation reaction (HOR), hydrogen evolution reaction (HER), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), nitrogen reduction reaction (NRR), nitrate reduction reaction (NO3−RR), and carbon dioxide reduction reaction (CO2RR). At the end, future research directions are provided to maximize the potential of leveraging the cation effects in the design of more efficient electrolyte systems.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1A. Groß, S. Sakong, Chem. Rev. 2022, 122, 10746–10776.
- 2H. Khani, A. R. Puente Santiago, T. He, Angew. Chem. Int. Ed. 2023, 135, e202306103.
10.1002/ange.202306103 Google Scholar
- 3S. Luo, C. Dai, Y. Ye, Q. Wu, J. Wang, X. Li, S. Xi, Z. J. Xu, Angew. Chem. Int. Ed. 2024, 63, e202402184.
- 4T. Wu, J. Ge, Q. Wu, X. Ren, F. Meng, J. Wang, S. Xi, X. Wang, K. Elouarzaki, A. Fisher, Z. J. Xu, Proc. Natl. Acad. Sci. USA 2024, 121, e2318652121.
- 5Y. Zhang, Q. Wu, J. Z. Y. Seow, Y. Jia, X. Ren, Z. J. Xu, Chem. Soc. Rev. 2024, 53, 8123–8136.
- 6F. Meng, Q. Wu, K. Elouarzaki, S. Luo, Y. Sun, C. Dai, S. Xi, Y. Chen, X. Lin, M. Fang, X. Wang, D. Mandler, Z. J. Xu, Sci. Adv. 2023, 9, eadh9487.
- 7Z. Zhang, H. Li, Y. Shao, L. Gan, F. Kang, W. Duan, H. A. Hansen, J. Li, Nat. Commun. 2024, 15, 612.
- 8J. Resasco, L. D. Chen, E. Clark, C. Tsai, C. Hahn, T. F. Jaramillo, K. Chan, A. T. Bell, J. Am. Chem. Soc. 2017, 139, 11277–11287.
- 9X. Qin, T. Vegge, H. A. Hansen, ACS Catal. 2024, 14, 8168–8175.
- 10Y. Yuan, J. Li, Y. Zhu, Y. Qiao, Z. Kang, Z. Wang, X. Tian, H. Huang, W. Lai, Angew. Chem. Int. Ed. 2025, e202425590.
- 11Y. Dong, M. Ma, Z. Jiao, S. Han, L. Xiong, Z. Deng, Y. Peng, Chinese Chem. Lett. 2024, 35, 109049.
- 12S. Ringe, Curr. Opin. Electrochem. 2023, 39, 101268.
- 13P. Xu, R. Wang, H. Zhang, V. Carnevale, E. Borguet, J. Suntivich, J. Am. Chem. Soc. 2024, 146, 2426–2434.
- 14X. Qin, T. Vegge, H. A. Hansen, J. Am. Chem. Soc. 2023, 145, 1897–1905.
- 15H. Jia, N. Yao, C. Yu, H. Cong, W. Luo, Angew. Chem. Int. Ed. 2023, 135, e202313886.
- 16V. J. Ovalle, Y. S. Hsu, N. Agrawal, M. J. Janik, M. M. Waegele, Nat. Catal. 2022, 5, 624–632.
- 17Y. Xu, Z. Xia, W. Gao, H. Xiao, B. Xu, Nat. Catal. 2024, 7, 1120–1129.
- 18P. Li, Y. Jiao, J. Huang, S. Chen, JACS Au 2023, 3, 2640–2659.
- 19A. S. Malkani, J. Anibal, X. Chang, B. Xu, iScience 2020, 23, 101776.
- 20J. A. D. del Rosario, G. Li, M. F. M. Labata, J. D. Ocon, P. Y. A. Chuang, Appl. Catal. B: Environ. 2021, 288, 119981.
- 21G. F. Li, M. Divinagracia, M. F. Labata, J. D. Ocon, P. Y. A. Chuang, ACS Appl. Mater. Interfaces 2019, 11, 33748–33758.
- 22Q. Ren, L. Xu, M. Lv, Z. Zhang, Z. Li, M. Shao, X. Duan, Chinese J. Catal. 2024, 63, 16–32.
- 23B. Huang, R. R. Rao, S. You, K. Hpone Myint, Y. Song, Y. Wang, W. Ding, L. Giordano, Y. Zhang, T. Wang, S. Muy, Y. Katayama, J. C. Grossman, A. P. Willard, K. Xu, Y. Jiang, Y. Shao-Horn, JACS Au 2021, 1, 1674–1687.
- 24Y. H. Wang, S. Zheng, W. M. Yang, R. Y. Zhou, Q. F. He, P. Radjenovic, J. C. Dong, S. Li, J. Zheng, Z. L. Yang, G. Attard, F. Pan, Z. Q. Tian, J. F. Li, Nature 2021, 600, 81–85.
- 25X. Y. Li, T. Wang, Y. C. Cai, Z. D. Meng, J. W. Nan, J. Y. Ye, J. Yi, D. P. Zhan, N. Tian, Z. Y. Zhou, S. G. Sun, Angew. Chem. Int. Ed. 2023, 135, e202218669.
- 26S. Yu, H. Yamauchi, S. Wang, A. Aggarwal, J. Kim, K. Gordiz, B. Huang, H. Xu, D. J. Zheng, X. Wang, H. Iriawan, D. Menga, Y. Shao-Horn, Nat. Catal. 2024, 7, 1000–1009.
- 27M. R. Gao, Y. C. Li, X. L. Zhang, X. L. Tai, X. P. Yang, P. C. Yu, S. C. Dong, L. P. Chi, Z. Z. Wu, Y. C. Zhang, S. P. Sun, P. G. Lu, L. Zhu, F. Y. Gao, Y. Lin, M. R. Gao, Angew. Chem. Int. Ed. 2025, e202422054.
- 28Z. Li, L. Wang, L. Sun, W. Yang, J. Am. Chem. Soc. 2024, 146, 23901–23908.
- 29L. Zhang, H. Hu, C. Sun, D. Xiao, H. T. Wang, Y. Xiao, S. Zhao, K. H. Chen, W. X. Lin, Y. C. Shao, X. Wang, C. W. Pao, L. Han, Nat. Commun. 2024, 15, 7179.
- 30X. Mao, X. Bai, G. Wu, Q. Qin, A. P. O'Mullane, Y. Jiao, A. Du, J. Am. Chem. Soc. 2024, 146, 18743–18752.
- 31S. Zheng, X. Yang, Z. Z. Shi, H. Ding, F. Pan, J. F. Li, J. Am. Chem. Soc. 2024, 146, 26965–26974.
- 32W. Liu, M. Xia, C. Zhao, B. Chong, J. Chen, H. Li, H. Ou, G. Yang, Nat. Commun. 2024, 15, 3524.
- 33M. C. Monteiro, F. Dattila, B. Hagedoorn, R. García-Muelas, N. López, M. T. Koper, Nat. Catal. 2021, 4, 654–662.
- 34M. Liu, Y. Pang, B. Zhang, P. D. Luna, O. Voznyy, J. Xu, X. Zheng, C. T. Dinh, F. Fan, C. Cao, F. P. G. Arquer, T. S. Safaei, A. Mepham, A. Klinkova, E. Kumacheva, T. Filleter, D. Sinton, S. O. Kelley, E. H. Sargent, Nature 2016, 537, 382–386.
- 35X. Qin, J. Li, T. W. Jiang, X. Y. Ma, K. Jiang, B. Yang, S. Che, W. B. Cai, Nat. Commun. 2024, 15, 7509.
- 36Q. Zhang, C. B. Musgrave, Y. Song, J. Su, L. Huang, L. Cheng, G. Li, Y. Liu, Y. Xin, Q. Hu, G. Ye, H. Shen, X. Wang, B. Z. Tang, W. A. Goddard, III, R. Ye, Nat. Synth. 2024, 3, 1231–1242.
- 37S. Ringe, E. L. Clark, J. Resasco, A. Walton, B. Seger, A. T. Bell, K. Chan, Energy Environ. Sci. 2019, 12, 3001–3014.
- 38A. S. Zeraati, F. Li, T. Alkayyali, R. Dorakhan, E. Shirzadi, F. Arabyarmohammadi, C. P. O'Brien, C. M. Gabardo, J. Kong, A. Ozden, M. Zargartalebi, Y. Zhao, L. Fan, P. Papangelakis, D. Kim, S. Park, R. K. Miao, J. P. Edwards, D. Young, A. H. Ip, E. H. Sargent, D. Sinton, Nat. Synth. 2025, 4, 75–83.
- 39J. Gu, S. Liu, W. Ni, W. Ren, S. Haussener, X. Hu, Nat. Catal. 2022, 5, 268–276.
- 40Z. Cui, A. J. W. Wong, M. J. Janik, A. C. Co, Sci. Adv. 2025, 11, eadr6465.
- 41M. R. Singh, Y. Kwon, Y. Lum, J. W. Ager, III, A. T. Bell, J. Am. Chem. Soc. 2016, 138, 13006–13012.
- 42S. J. Shin, H. Choi, S. Ringe, D. H. Won, H. S. Oh, D. H. Kim, T. Lee, D. H. Nam, H. Kim, C. H. Choi, Nat. Commun. 2022, 13, 5482.
- 43J. Liu, H. Yin, P. Liu, S. Chen, S. Yin, W. Wang, H. Zhao, Y. Wang, J. Phys. Chem. Lett. 2019, 10, 6955–6961.
- 44P. Li, Y. Jiang, Y. Hu, Y. Men, Y. Liu, W. Cai, S. Chen, Nat. Catal. 2022, 5, 900–911.
- 45B. Tang, Y. Fang, S. Zhu, Q. Bai, X. Li, L. Wei, Z. Li, C. Zhu, Chem. Sci. 2024, 15, 7111–7120.
- 46Y. H. Wang, Y. Yang, F. Y. Gao, X. L. Zhang, L. Zhu, H. K. Yan, P. P. Yang, M. R. Gao, Angew. Chem. Int. Ed. 2024, 63, e202407613.
- 47P. Li, Y. L. Jiang, Y. Men, Y. Z. Jiao, S. Chen, Nat. Commun. 2025, 16, 1844.
- 48A. H. Shah, Z. Zhang, Z. Huang, S. Wang, G. Zhong, C. Wan, A. N. Alexandrova, Y. Huang, X. Duan, Nat. Catal. 2022, 5, 923–933.
- 49E. Liu, J. Li, L. Jiao, H. T. T. Doan, Z. Liu, Z. Zhao, Y. Huang, K. M. Abraham, S. Mukerjee, Q. Jia, J. Am. Chem. Soc. 2019, 141, 3232–3239.
- 50R. R. Rao, B. Huang, Y. Katayama, J. Hwang, T. Kawaguchi, J. R. Lunger, J. Peng, Y. Zhang, A. Morinaga, H. Zhou, H. You, Y. Shao-Horn, J. Phys. Chem. C 2021, 125, 8195–8207.
- 51K. Zhao, M. Luo, Y. Zhang, X. Chang, B. Xu, Nat. Catal. 2025, 8, 46–57.
- 52D. Strmcnik, K. Kodama, D. Van der Vliet, J. Greeley, V. R. Stamenkovic, N. M. Marković, Nat. Chem. 2009, 1, 466–472.
- 53C. Yang, O. Fontaine, J. M. Tarascon, A. Grimaud, Angew. Chem. Int. Ed. 2017, 129, 8778–8782.
- 54A. C. Garcia, T. Touzalin, C. Nieuwland, N. Perini, M. T. M. Koper, Angew. Chem. Int. Ed. 2019, 58, 12999–13003.
- 55C. Yang, O. Fontaine, J.-M. Tarascon, A. Grimaud, Angew. Chem. Int. Ed. 2017, 56, 8652–8656.
- 56M. Görlin, J. Halldin Stenlid, S. Koroidov, H. Y. Wang, M. Börner, M. Shipilin, A. Kalinko, V. Murzin, O. V. Safonova, M. Nachtegaal, A. Uheida, J. Dutta, M. Bauer, A. Nilsson, O. Diaz-Morales, Nat. Commun. 2020, 11, 6181.
- 57S. G. Ji, M. M. Kim, M. H. Han, J. Cho, Y. Son, Y. Kim, J. Jeong, Z. Kim, K. Chae, H. Oh, H. Kim, C. Choi, Nat. Catal 2024, 7, 1330–1338.
- 58S. Han, H. Li, T. Li, F. Chen, R. Yang, Y. Yu, B. Zhang, Nat. Catal. 2023, 6, 402–414.
- 59Q. Wu, C. Dai, F. Meng, Y. Jiao, Z. J. Xu, Nat. Commun. 2024, 15, 1095.
- 60Y. C. Hao, Y. Guo, L. W. Chen, M. Shu, X. Y. Wang, T. A. Bu, W. Y. Gao, N. Zhang, X. Su, X. Feng, J. W. Zhou, B. Wang, C. W. Hu, A. X. Yin, R. Si, Y. W. Zhang, C. H. Yan, Nat. Catal. 2019, 2, 448–456.
- 61W. Wen, S. Fang, Y. Zhou, Y. Zhao, P. Li, X. Y. Yu, Angew. Chem. Int. Ed. 2024, 63, e202408382.
- 62X. Qin, H. A. Hansen, K. Honkala, M. M. Melander, Nat. Commun. 2023, 14, 7607.
- 63F. Wu, X. Liu, S. Wang, L. Hu, S. Kunze, Z. Xue, Z. Shen, Y. Yang, X. Wang, M. Fan, H. Pan, X. Gao, T. Yao, Y. Wu, Nat. Commun. 2024, 15, 6972.
- 64Q. Zhu, C. L. Rooney, H. Shema, C. Zeng, J. A. Panetier, E. Gross, H. Wang, L. R. Baker, Nat. Catal. 2024, 7, 987–999.
- 65H. M. Yan, Z. Zhang, Y. G. Wang, ACS Catal. 2024, 14, 3596–3605.
- 66X. Yang, H. Ding, S. Li, S. Zheng, J. F. Li, F. Pan, J. Am. Chem. Soc. 2024, 146, 5532–5542.
- 67M. C. Monteiro, F. Dattila, N. López, M. T. Koper, J. Am. Chem. Soc. 2022, 144, 1589–1602.
- 68E. Sargeant, P. Rodriguez, F. Calle-Vallejo, ACS Catal. 2024, 14, 8814–8822.
- 69F. Y. Chen, A. Elgazzar, S. Pecaut, C. Qiu, Y. Feng, S. Ashokkumar, Z. Yu, C. Sellers, S. Hao, P. Zhu, H. Wang, Nat. Catal. 2024, 7, 1032–1043.
- 70X. Wang, H. Zhang, G. Wei, J. Xing, S. Chen, X. Quan, Sci. Adv. 2024, 10, eado3998.