Revealing the Nature of Non-Covalent Interactions in Ionic Liquids by Combined Pulse EPR and 19F NMR Spectroscopy
Graphical Abstract
Ionic liquids have been proposed as alternatives to molecular solvents. Here, spin probes and spin-labelled ILs are studied by pulse electron paramagnetic resonance, paramagnetic relaxation enhancement NMR, molecular dynamics, and density functional theory, revealing the IL interaction sphere. The results challenge previous interpretations of nitroxide-solvent nanostructuring in ILs.
Abstract
Ionic liquids (ILs) are a unique class of compounds that have attracted interest for numerous and diverse applications, ranging from solvents for sustainable synthesis to sustainable electrolytes. Understanding their nanostructure and solute-solvent interactions is a prerequisite to harnessing the full potential of ILs. It has been proposed that ILs solvate non-polar solutes via their alkyl chains through the formation of nanoscale structures, such as micelles. Here, we determine the non-covalent interactions responsible for such nanostructuring in ILs. We use pulse electron paramagnetic resonance (EPR), paramagnetic relaxation enhancement (PRE) NMR, molecular dynamics (MD), and density functional theory (DFT) calculations in combination with ILs tailored to probe specific interactions through spin and isotopic labelling. Inter- and intramolecular cation–anion interactions are probed by electron-nuclear double resonance (ENDOR) and 19F PRE experiments and show that nitroxide solutes associate with the polar domains of the imidazolium cation through weak hydrogen bonding with the imidazolium ring protons, as supported by MD simulations. Thus, this study reveals a less structured nanostructure than a micellar picture might suggest, but with clear IL cation-solute interactions. Our methodology to reveal nanostructure not only has implications for ILs but is also applicable to other soft matter systems.
Introduction
Ionic liquids (ILs) are systems composed entirely of ions that are liquid at the temperature of interest; they are solvents with properties distinct from those of conventional molecular solvents.[1, 2] In recent years, the study of ILs has expanded to almost every aspect of chemical research, ranging from non-aqueous electrolytes[3] and carbon capture[4] to biological applications such as protein crystallisation[5, 6] and drug delivery.[7, 8]
Much attention has focused on the structure and dynamics of imidazolium-based ILs.[9, 10] These ILs feature an organic or inorganic anion, and an organic amphiphilic cation, consisting of a polar imidazolium core and a non-polar alkyl chain. The diverse choice of anionic components and the degree of cationic alkylation imparts tunability and distinct physicochemical properties to these ILs. Hence, ILs are often called “designer solvents”,[11] particularly with regard to their use in organic chemistry.[12] Imidazolium-based ILs are typically non-volatile and non-flammable, facilitating their recovery and reuse.[13, 14] ILs have therefore also been demonstrated to enhance the sustainability of many chemical processes.[14-16] Their application as alternatives to aqueous electrolytes arises from their non-flammability, their large electrochemical windows, and favorable conductance properties.[17] Indeed, recent computational and experimental studies demonstrated enhanced electrochemical control by judicious choice of IL-based electrolytes.[18, 19]
The unique physicochemical properties of ILs arise because of their structures and the solvent environments they present.[20] Although ILs share some similarities with other complex liquid systems, such as molten salts, liquid crystals, and glasses, the combination of physicochemical properties are unique to ILs.[21-24] It is well known that ILs have significant nanostructuring in the liquid state, with segregation of the imidazolium cation into polar and non-polar domains, as determined primarily by molecular dynamics (MD) simulations and small-angle X-ray scattering techniques.[25, 26] Solute–solvent interactions in ILs have also attracted much interest.[27] In addition to the ubiquitous Coulombic interactions, hydrogen bonding, π–π interactions and dispersion interactions have all been found to be significant.[28] These interactions are dependent upon the complementarity of the solute and the ILs’ ions, e.g. hydrogen bond donor solute with hydrogen bond acceptor anion, hydrogen bond acceptor solute with hydrogen bond donor cation, π–π interactions between aromatic solutes and imidazolium ions, and dispersion interactions between non-polar solutes and long alkyl chain cations.[29] Despite the fact that ILs have been intensively studied since the early 1990's, widespread industrial applications are still limited.[30] One bottleneck is the lack in understanding of how inter- and intra- molecular interactions lead to macroscopic properties of ILs, making process optimisation difficult.[31] Therefore studies on the structure and dynamics of ILs are more relevant than ever, as understanding the fundamental properties of ILs is key to their large-scale applications. In addition to deepening understanding of ILs, findings from their chemistry can be transferred to other soft matter systems such as polymers, bio-membranes, gels, liquid crystals, and nanocolloids. Despite the intense research activity aimed at understanding IL nanostructuring, solute dynamics, and solute–solvent interactions, these have largely been parallel activities and have not yet been brought together in a single study. Strikingly, the nature of the dynamic equilibrium between the nanodomains of solutes that experience both the ionic and the non-polar nanodomains, and interfacial phenomena between these, remains unclear.
Magnetic resonance techniques are ideally suited to report on the heterogeneity of the IL nanoenvironment, nanostructure, equilibria dynamics and solute–solvent interactions therein. To date, continuous wave (CW) electron paramagnetic resonance (EPR) and magnetic relaxation measurements have predominantly been employed to probe the environment and dynamics of imidazolium-based IL nanodomains using paramagnetic spin probes, primarily nitroxide-based radicals,[32-35] but also photoexcited triplet states.[36, 37] CW studies have given insight to the microviscosity of the IL nanoenvironment,[38] while field-dependent relaxation measurements showed that the librational motions of various spin probes undergo an anomalous suppression around the ILs’ glass transition temperature (Tg). This feature is ubiquitous in imidazolium-based ILs and alkyl chain-bearing non-IL glasses, and is found to be spin probe independent, but dependent on the alkyl chain length.[39-42] Together with MD results it has therefore been suggested that the studied spin probes are generally located in a micelle-like environment and solvated at the interface of the non-polar alkyl chains of the ILs.[43] EPR studies of IL self-probes, i.e., paramagnetic ILs, reported directly on the heterogeneity of an all-IL nanoenvironment and show close to ideal mixing, minimising disruptions from using non-IL probes.[44, 45] Notably, organic nitroxide-based ILs show equal promise for spectroscopic investigations of ILs and as efficient electrolytes and redox mediators,[46] or recoverable solvent-free catalysts.[47]
Pulse hyperfine electron EPR spectroscopy is a suite of techniques that offers unparalleled sensitivity in revealing the local structure around unpaired electrons through their interactions with surrounding magnetically active nuclei.[48] While pulse hyperfine methods have found great success in fields ranging from surface catalysis[49, 50] to structural biology,[51, 52] they have not yet been applied to IL systems. Of particular relevance are 19F Mims electron-nuclear double resonance (ENDOR)-based distance measurements in the 5–20 Å range that exploit the dipolar interaction between an unpaired electron and 19F nuclear spins. This has been recently demonstrated at W-band (ca. 94 GHz) in model systems,[53, 54] proteins,[55–-57] in-cell,[58] and also at more accessible Q-band frequencies (ca. 34 GHz).[59] Further, applications of 2H Mims ENDOR are attractive for elucidation of the H-bonding environment in (per)deuterated samples.[60, 61] This is facilitated by the hyperfine coupling values, and the dependence of the nuclear quadrupole coupling constant on the hybridisation of the 2H nuclear spin centre.[62] We reasoned that in ILs it should thus be possible to employ Mims ENDOR methods to detect coupled nuclei to a solvated paramagnetic centre, thereby reporting on the structure of the local nuclear environment. Paramagnetic relaxation enhancement (PRE) NMR can also access electron-nuclear distances, with the advantage that samples are measured in the liquid state (in contrast to the ENDOR measurements that are typically performed in frozen solutions <100 K).[63, 64] Although not yet applied to ILs, PRE NMR of 19F nuclear spins has been demonstrated as a sensitive method for room temperature biomolecular structural determination in the 9–30 Å distance range.[65-67] PRE NMR has also served as a validation tool for solid-state ENDOR distance measurements.[58, 68]
In this work, we prepared nitroxide-based spin probes dissolved in imidazolium-based ILs (Figure 1) alongside a family of nitroxide spin-labelled ILs dissolved in conventional molecular solvents, or in imidazolium-based ILs. We apply pulse hyperfine EPR spectroscopy in combination with PRE NMR to investigate the non-covalent interactions in ILs using both spin probes and spin-labelled ILs. We determine the cation-anion interaction strengths and the solute–solvent H-bonding environment, providing detailed insight into the IL solvation nanostructure in the solid and liquid state. Density functional theory (DFT) calculations and MD simulations complement our magnetic resonance results. The toolkit of pulse hyperfine EPR, PRE NMR and computational techniques, shown here to elucidate IL solvation nanostructure and dynamics, thus paves the way to determine nanostructural organisation, equilibria dynamics and heterogeneity in similar semi-structured soft matter systems.
Results and Discussion
Interactions of Spin Probes Dissolved in ILs
Much of the current literature focuses on the interaction of spin probes with the cationic component of ILs. However, it is well known that the anionic component is highly relevant in affording different physicochemical properties, including reactivity, acidity/basicity, hydrophobicity, and viscosity.[20, 69, 70] Thus, we first determined the IL anionic environment of 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) as a spin probe dissolved in [C2C1im]+-based ILs with different anions, namely [BF4]−, [NMes2]−, [NTf2]− and [FSI]− (Figure 2 and Supporting Information S.1 for synthetic details). Anions were selected based on the following criteria: a) they are each “weakly-coordinating”,[71] making a proof-of-concept study more feasible due to fewer possible interactions; b) they have large electrochemical windows, thermal and chemical stability, and thus present great interest for electrochemical and battery applications;[72, 73] c) the synthetic methodology is well-developed and results in high purity ILs, an important consideration for pulse EPR measurements, where signals from impurities could be detrimental.[74]
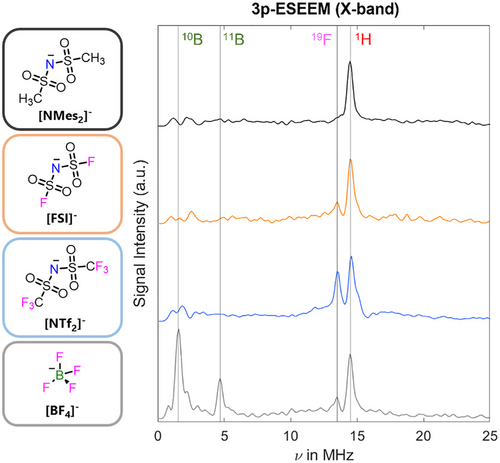
The echo-detected field sweep (EDFS) spectra of TEMPOL dissolved in each IL were similar for all anions studied (Supporting Information S.2 and Figure S1). In order to gather information on the TEMPOL environment, 3-pulse electron spin echo envelope modulation (3p-ESEEM) experiments at X-band were performed, detecting on the maximum of the TEMPOL signal at 50 K (Figures 2, S.2 and S.3 for details). The processed ESEEM spectrum showed distinct 1H peaks centred around their nuclear Larmor frequency for all systems, corresponding to the hydrogen atoms of both the cationic and anionic components of the studied ILs. For the [FSI]− and [NTf2]− anions, a 19F peak is observed which increases in intensity for the latter, due to an increasing number of 19F nuclei interacting with the TEMPOL. For the [BF4]− sample the most pronounced peak seen is centred about the 10B Larmor frequency, while the 11B nuclear isotope is lower in intensity, and the 19F peak is suppressed. In all cases, it is evident that the spin probe has a hyperfine interaction with both the IL cation and anion. Based on the 3p-ESEEM investigation, we decided to focus on the [NTf2]− anion. First, the terminal trifluoromethyl (-CF3) groups give rise to intense signals. Second, they offer the possibility to determine the cation-anion interaction strength based on distances between the nitroxide spin centre and the [NTf2]− anion using 19F Mims ENDOR.[53]
Spin Probes and Spin-labelled ILs
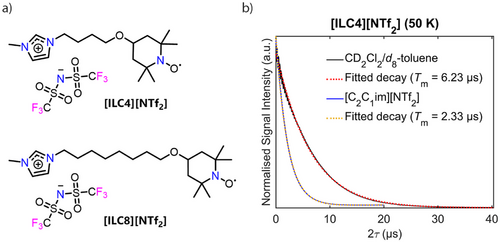
Much of the structural research on ILs has been based on indirect measurements of model solutes, using a variety of techniques including infrared,[75] Raman[76] and 1H NMR.[77] Welton et al. have studied the UV–vis spectra of organic dyes in ILs as a measure of their polarity extensively.[78, 79] However, the selection of the dye can influence the measured polarity values,[70, 80] indicating that the functionality of the solute can cause preferential solvation by the IL ions around it and hence influence the perceived polarity. Most existing IL-related EPR studies focus on nitroxide-based spin-probes, such as TEMPOL. Although spin probes are extremely useful reporters of the IL nanoenvironment, it is not clear to what extent the use of a non-IL probe impacts the nanostructuring of ILs. Spin-labelling methods, akin to those used in the structural determination of biomolecules,[81] applied to IL systems have the potential to allow direct measurements of an all-IL nanoenvironment. There is only one prior EPR study employing spin-labelled ILs, which demonstrated that non-IL spin probe host-guest type interactions with ILs are not responsible for the nanostructuring related to the anomalous suppression of molecular mobility around Tg.[45] Thus, in order to determine the non-covalent interactions governing heterogeneity in an all-IL system, the spin-labelled ILs, [ILC4][NTf2] and [ILC8][NTf2] were synthesised (Figure 3a). The nitroxide spin-labelled ILs are easily accessible, metal-free, room temperature ILs. They exhibit characteristic nitroxide EDFS spectra (Figure S3) and a relatively long phase memory time (Tm) at 50 K when dissolved in deuterated molecular solvents, as well as in ILs (Figure 3b).
Cation-Anion Associations Within Spin-labelled ILs
Ionic compounds in solution experience different degrees of ionic association, depending on the polarity and the dielectric constant of the solvent. For molecular solvents this phenomenon is generally well understood, with high polarity solvents showing a low degree of ion association (free solvated ions and/or solvent-separated ion pairs), while low polarity solvents show a high degree of ion association (penetrated and/or contact ion pairs). Ion pairing in ILs is more complex and has been widely studied both computationally and experimentally.[82-84]
| Solvent matrix | IL | τ (µs) | lwa) (KHz) | Tsim1 ± σ (KHz) | Tsim2 ± σ (KHz) |
|---|---|---|---|---|---|
|
CD2Cl2/ d8-toluene |
[ILC4] [NTf2] | 2.2 | 20 | 105 ± 40 | 300 ± 28 |
|
CD2Cl2/ d8-toluene |
[ILC8] [NTf2] | 2.2 | 45 | 50 ± 35 | 220 ± 38 |
- a) Full-width half maximum (FWHM) of a convoluted Lorentzian lineshape, where the abscissa step was set to four.
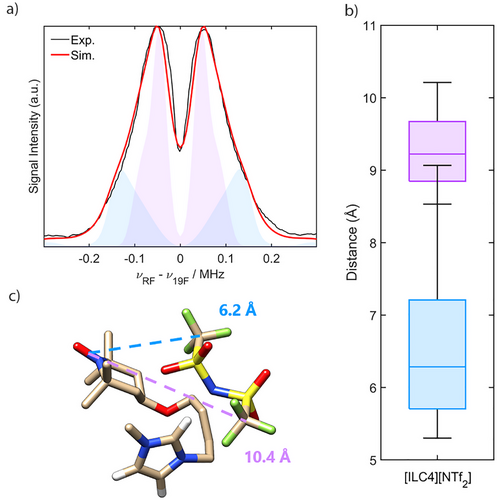
Samples of the diamagnetic reference, [C8C1im][NTf2], and the spin-labelled ILs, [ILC4][NTf2] and [ILC8][NTf2], in both CD2Cl2 and CD3CN solvent matrices each exhibited a single 19F resonance. This corresponds to the averaged trifluoromethyl moieties of the [NTf2]− anion, with remarkably similar chemical shift values (Table 2). The values of τr were approximated via simulation of the room temperature CW EPR spectra of the spin-labelled ionic liquids [ILC4][NTf2] and [ILC8][NTf2] in both CD2Cl2 and CD3CN (Figure S47). The determined values, τr = 0.1–0.12 ns, correspond to the expected sub-nanosecond fast-motion regime, demonstrating that there are no local viscosity effects owing to the spin-labelled ILs. Moreover, a small value of τr is expected for dilute solutes of low molecular weight systems in molecular solvents of low viscosity (at room temperature the measured viscosity of CD2Cl2 and CD3CN is 0.413 and 0.369 mPa s, respectively). The Γ2 rates were then determined from the Carr—Purcell—Meiboom–Gill (CPMG) spin-spin relaxation data to obtain the solution averaged nitroxide-19F distances for the spin-labelled ILs, shown in Table 2 (see Supporting Information S6 for details).[64] For both spin-labelled ILs, the PRE-derived values in CD2Cl2 corresponded approximately to the longer nitroxide-19F distances determined from 19F Mims ENDOR. This shows that flash-freezing of the spin-labelled ILs prior to pulse EPR measurements captures a representative picture of the cation–anion interactions in the solution state. The drawback of PRE NMR compared to ENDOR is that only one solution-averaged distance is obtained if only one 19F resonance is present at room temperature, and thus the calculated value represents the sum of each dynamic nitroxide-19F intramolecular interaction per spin-labelled IL. Samples measured in CD3CN exhibited considerably longer PRE-derived distances (Table 2). Noting that CD3CN and CD2Cl2 have room-temperature dielectric constants of 37.5 and 8.93, respectively, the longer distances in CD3CN demonstrate that solvents with a higher dielectric constant have a greater capacity to separate the cation–anion interactions, as previously suggested by DFT calculations[87] and observed by conductivity measurements and vibrational spectroscopy.[88-90] PRE NMR therefore represents an alternative approach to determining the room temperature strength of long-lived ionic associations by NMR, where nuclear Overhauser effect (NOE) experiments, or careful examination of chemical shifts under titration, or of pseudocontact shifts in the case of lanthanide-based ILs, have thus far been employed exclusively.[88, 91-93]
| Solvent matrix | IL | Chemical shift (ppm) | Γ2 (s−1) | r (Å) |
|---|---|---|---|---|
| CD2Cl2 | [C8C1im][NTf2] | −79.55 | – | – |
| CD2Cl2 | [ILC4][NTf2] | −79.21 | 19.4 | 8.8 |
| CD2Cl2 | [ILC8][NTf2] | −79.38 | 7.9 | 10.3 |
| CD3CN | [C8C1im][NTf2] | −80.20 | – | – |
| CD3CN | [ILC4][NTf2] | −80.11 | 1.4 | 13.5 |
| CD3CN | [ILC8][NTf2] | −80.16 | 1.4 | 13.5 |
Nitroxide-Anion Interactions are Independent of the Cation
With a detailed understanding of the molecular interactions that occur within isolated spin-labelled IL ion pairs, we turned to investigating the solute–solvent interaction sphere of [ILC4][NTf2] and [ILC8][NTf2] dissolved in different IL systems (rather than traditional solvents), namely [C2C1im][NTf2], [C8C1im][NTf2], and [C8C1C1im][NTf2]. While measurements with [C2C1C1im][NTf2] were attempted, this IL undergoes a phase transition from liquid to solid around room temperature and was therefore not investigated further (see Supporting Information S3). By monitoring the dipolar interaction of the spin-labelled IL with the 19F nuclei of the [NTf2]− anion, we can monitor changes in the IL solvation nanostructure as the IL environment is altered. The 19F Mims ENDOR experiments of [ILC4][NTf2] dissolved in these ILs showed a broad distribution of dipolar coupling interactions. However, there were no major changes in these interactions between the different IL matrices (Figure 5a), which were broadened compared to the isolated spin-labelled IL ion pairs in molecular solvents (Figure 4a). A similar set of results was obtained for [ILC8][NTf2] (Figure S14).
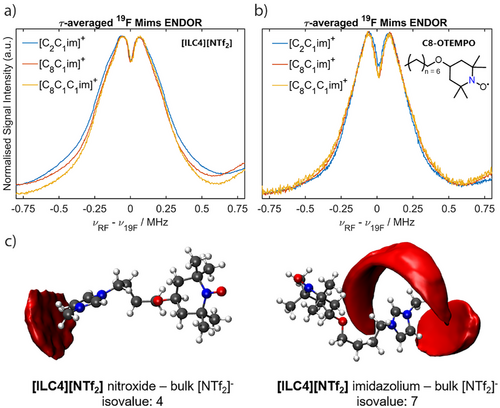
Determination of the experimental hyperfine coupling value from the spectral splitting, Tread, allows an upper distance limit for the anionic solvation shell to be obtained. The anionic solvation shell surrounding the spin centree is corroborated by MD results taking 50 [ILC4]+ spin-labelled cations in 450 [C8C1im]+ cations and 500 [NTf2]− anions (details in Supporting Information S4). The spatial distribution functions (SDFs) show a preferential interaction of the bulk [NTf2]− anions with both the [C8C1im]+ and the spin-labelled IL imidazolium ring via an electrostatic interaction. No short-range interactions between the [NTf2]− anion and the nitroxide spin centre are observed (Figure 5c). Therefore, from both the MD simulations and the ENDOR spectra, we conclude that the anionic solvation shell is independent of the IL cation structure, with an anionic solvation shell size with respect to the electron spin of ca. 9.5 nm. Having shown that the [NTf2]− anion's interaction with the spin-labelled cations is not dependent on the IL cation structure, we sought to determine if the same results are observed for uncharged spin probes. We therefore repeated the 19F Mims ENDOR measurements using the synthesised uncharged spin probe C8-OTEMPO (synthetic details available in Supporting Information S1), dissolved in the same ILs (Figure 5b). The anionic interactions with the spin probe are again the same for all IL cations. The observed couplings are less broad compared to the spin-labelled ILs, due to the lack of an intramolecular 19F coupling from an associated [NTf2]− anion with the spin probe. Experiments in the same IL systems using the TEMPOL spin probe gave similar results (Figure S16). Thus, the spin centre of each solute (spin-labelled ILs, C8-OTEMPO and TEMPOL) exhibits primarily long-range interactions with the IL anion.
Nitroxides Preferentially Associate with the Imidazolium Ring
Q-band 1H Mims ENDOR spectra were measured to understand the local 1H spin density qualitatively. Considering [ILC4][NTf2] dissolved in [C2C1im][NTf2], [C8C1im][NTf2] and [C8C1C1im][NTf2], an additional weak coupling distribution is observed in the [C2C1im][NTf2] 1H Mims ENDOR spectrum, compared to the [C8C1im][NTf2] and [C8C1C1im][NTf2] spectra (Figure 6a). This is postulated to be due to the nitroxide spin-centre coupling to hydrogens located in the 2nd solvation shell of [C2C1im][NTf2], whose smaller molecular volume can pack closer to the spin centre compared to ILs with long alkyl chains. To probe interactions with the cations further, we perdeuterated the cations’ imidazolium ring to give [d3-C2C1im][NTf2] and [d3-C8C1im][NTf2] ILs (synthetic details in Supporting Information S1). [ILC4][NTf2] dissolved in the perdeuterated ILs showed a marginally weaker distribution of 1H couplings compared to the non-deuterated samples, most evident in the [d3-C2C1im][NTf2] sample (Figure 6b).
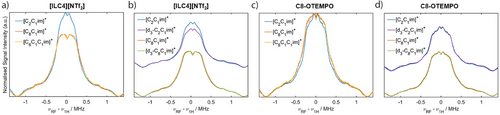
The same results are observed for the longer chain spin-labelled IL [ILC8][NTf2] (Figure S19). For the spin probe C8-OTEMPO dissolved in [C2C1im][NTf2], a similar 1H ENDOR spectrum is recorded as for the spin-labelled ILs, however the [C8C1im][NTf2] and [C8C1C1im][NTf2] samples show a change in the 1H environment that is different to the change observed in the spin-labelled ILs (Figure 6c). C8-OTEMPO dissolved in perdeuterated ILs showed a less evident change in 1H couplings compared to the non-deuterated samples (Figure 6d). These results suggest that both the spin-labelled ILs and spin probes experience small changes in the 1H solvation environment between ILs beyond the first coordination sphere where the interactions are expected to be weak (>1 MHz). Recently reported intermolecular hyperfine relaxation-induced dipolar modulation enhancement (ih-RIDME) experiments may be a useful tool to quantitatively describe the 1H environment of different spin probes dissolved in ILs.[94]
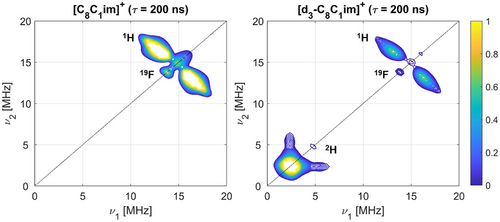
X-band 4-pulse hyperfine sublevel correlation (HYSCORE) experiments (experimental details in Supporting Information S2) also showed only weak couplings at the 1H Larmor frequency, with no appreciable difference recorded between the ILs. A broad 1H coupling distribution of ca. |2T + aiso| = 8 MHz can be assigned from the HYSCORE spectrum in all cases. HYSCORE measurements of the spin-labelled ILs dissolved in perdeuterated ILs revealed an additional feature, corresponding to a weakly coupled single quantum (SQ) peak centred at the 2H Larmor frequency alongside a weaker double quantum (DQ) peak, stemming from the small, positive quadrupolar moment of 2H (Figure 7). 6-pulse HYSCORE measurements, which suffer less from multi-nuclear suppression effects,[95] were almost indistinguishable (Figure S22). 4-pulse HYSCORE measurements under the same conditions using the spin-probe C8-OTEMPO showed similar 1H coupling distributions and the appearance of 2H signals in the perdeuterated samples (Figure S23). Together with the ENDOR measurements, these results point to the nitroxide spin centre in all samples preferentially interacting with the imidazolium ring hydrogens rather than the alkyl chain hydrogens.
Weak Hydrogen Bonding is Observed with Nitroxide Solutes
To assign the 2H peaks stemming from the perdeuterated imidazolium ring observed in the X-band HYSCORE measurements, we probed the 2H nuclei directly using Mims ENDOR (Supporting Information S2 for details). Nitroxide spin centres are known hydrogen bond acceptors.[96-98] The hydrogen atoms of the imidazolium ring have been shown to be the most accessible protons for hydrogen bonding in imidazolium-based ILs.[70, 88, 99] Therefore with 2H Mims ENDOR, we aimed to determine if the nitroxide spin centre is interacting with the hydrogen atoms of the imidazolium ring, and if the cation structure has any effect on this interaction. For the spin-labelled IL [ILC4][NTf2], a coupling of the nitroxide spin centre to 2H nuclei is observed that does not change in magnitude between [d3-C2C1im][NTf2] and [d3-C8C1im][NTf2] (Figure 8a). Simulations of the individual 2H ENDOR data sets were performed excluding the matrix peak at the 2H Larmor frequency and reasonable fits to the data were obtained with T = 0.6–0.8 MHz (Figure S24), assuming a purely dipolar interaction (i.e., A = [-T, -T, 2T]).[100] The inclusion of nuclear quadrupole parameters had no effect on the fitting of the ENDOR simulations. This is likely due to the small magnitude of both the quadrupole coupling constant, e2Qq/h ≈ 150 – 200 KHz, and asymmetry parameter, η < 0.1, expected for rigid sp2 hybridised C─D bonds.[61] In conjunction with Q-band 1H Davies ENDOR difference spectra of TEMPOL in protonated and deuterated ILs (Figure S25), the observed hyperfine coupling interactions can be attributed to weak hydrogen bonding of the nitroxide spin centre with all three of the 2H atoms of the imidazolium ring. The inter-atomic D⋯O• lengths (2.0–2.7 Å) derived from the hyperfine couplings are approximate as they depend on the delocalisation of the electronic spin density ρ (see Figure 8b, and further details in Figure S26). Similar 2H Mims ENDOR spectra were observed for the spin labelled IL [ILC8][NTf2], and the spin probe samples, C8-OTEMPO and TEMPOL (Figure S28 and S29).
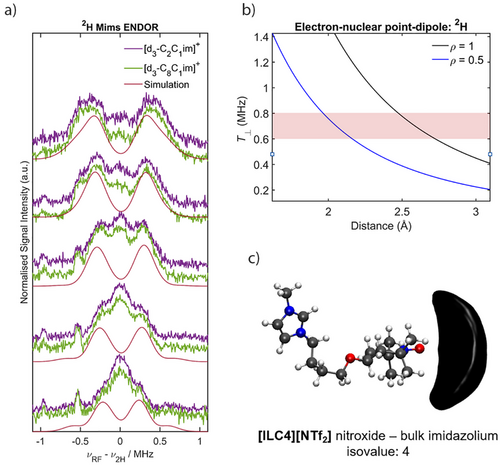
The hydrogen bonding interaction determined from the ENDOR experiments is further supported by MD simulations of [ILC4][NTf2] dissolved in [C8C1im][NTf2] (Figure 8c). Our MD results (Figure 9) show that the highest coordination numbers are present in systems with interactions predominantly driven by dispersion forces and clustering, as shown previously for nitroxide-based solutes.[101] This may be partially due to these solvation shells being slightly larger (Figure S35), however this does not explain the entirety of the relatively large coordination numbers. Previous results have demonstrated that the most energetically favorable ion pair orientations are those where the cations are stacked on top of each other and the anion lies off-plane from the cations, thus stabilising the charge.[29] Interestingly, the MD simulations suggest a preferential interaction of the nitroxide spin centre with the C4/C5 protons of the imidazolium ring over the C2 proton, possibly due to a cooperative bonding effect, as well as the higher surface area for the interaction.
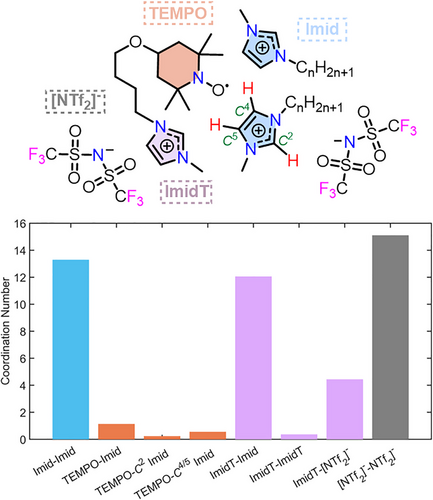
Crucially, the micelle-like IL solvation structure previously reported,[43] where nitroxide solutes have been proposed to preferentially associate with the non-polar alkyl chains of the IL cation, is not supported in this study, neither experimentally nor computationally. Rather, it is the polar imidazolium ring that is found to associate favorably with the solute, for both nitroxide spin probes and the spin-labelled ILs (Figure 9). Further, dynamic light scattering (DLS) experiments of [ILC4][NTf2] dissolved in [d3-C8C1im][NTf2] did not show any evidence of micellar nanostructuring around the nitroxide solutes at the concentrations used for pulse EPR measurements (see Supporting Information S7). Thus, the picture that emerges is a less ordered, or “softer”, solute–solvent nanostructure (Figure 9) than previously hypothesised.
Conclusion
We have applied a combination of advanced pulse hyperfine EPR techniques, 19F PRE NMR, DFT calculations and MD simulations in order to determine the solute–solvent interaction sphere and molecular level nanostructuring of imidazolium-based ILs. A family of spin probes and nitroxide spin-labelled ILs were synthesised, featuring targeted isotopic labelling, such that both the intra- and intermolecular non-covalent interactions could be measured by magnetic resonance techniques. The solvation of nitroxide radicals has previously been associated with the formation of micelle-like structures around the solute. Instead, our ENDOR results, supported by MD simulations, reveal that the solute–solvent nanostructures in imidazolium-based ILs do not form a micelle-like structure around nitroxide solutes, but rather are found to associate with the polar domains of the imidazolium cation through weak hydrogen bonding with the ring protons. The consistent long-range solvation of the nitroxide-based spin centres by the 19F nuclei of the anionic constituent of the ILs, independent of the cationic component, further support this revised picture of IL nanostructuring. Our results thus challenge current interpretations of nitroxide solute–solvent interactions in ILs and could have implications for the design and use of next-generation, task-specific IL systems. Further, the methodologies presented herein could be extended to characterise non-covalent interactions in similar soft matter systems.
Supporting Information
The authors have cited additional references within the Supporting Information.[18, 45, 74, 102-121] The raw data supporting this study, and the MATLAB-based data analysis and simulation routines, are available from the Imperial Research Data repository DOI: 10.14469/hpc/14504.
Acknowledgements
C.J.R., S.K., and J.E. acknowledge funding from the Leverhulme Trust–Research Grant reference RPG-2021–383. G.J.S. acknowledges funding from NIHR Health Protection Research Unit in Chemical and Radiation Threats and Hazards. C.J.R. and G.J.S. would like to acknowledge the Research Computing Service facilities at Imperial College London for access to the HPC cluster: Imperial College Research Computing Service, DOI: 10.14469/hpc/2232. Mr Peter Haycock (Imperial College London) is acknowledged for assistance with the 19F PRE NMR experiments. The EPR measurements were performed at the Centre for Pulse EPR (PEPR) at Imperial College London, supported by the EPSRC grant EP/T031425/1 to M.M.R. The authors thank Dr Alberto Collauto for technical support and helpful comments on the manuscript. Ms. Eleanor Clifford is thanked for assistance with running the DLS measurements. NMR spectra were recorded at the NMR facility of the Department of Chemistry at Imperial College London. L.W would like to thank the PC2 computational cluster of the University of Paderborn as well as the HPC clusters of the University of Bonn for computational resources.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.