Data-Driven Review on Aerogels and Polymeric Aerogels
Graphical Abstract
A review goes to data-driven. An exhaustive literature survey provides an overview of research status on aerogels, a category of highly porous solids with unique physicochemical properties. Statistical/data-driven analysis tells us positive/negative aspects; aerogels have a promising potential to contribute to emerging energy/environmental issues, while they are causing an academic fever with irresponsible exaggerations and a flood of unreliable data.
Abstract
A thoroughly data-driven review on the chemistry, properties, and applications of aerogels is provided. Aerogel is a category of solid materials having homogeneous, highly porous nanostructures. Unique physicochemical properties of aerogels have attracted much attention in wide fields due to their potentials for energy/environmental issues in the 21st century, while an exponentially increasing number of papers are causing an academic fever and unreliable data issues. Statistical analysis based on an exhaustive literature survey from almost all the papers with “aerogel” in their titles reveals a flood of freeze-dried aerogels mainly consisting of organic polymers and organic–inorganic composites. Process-property data from polymer aerogel papers are analyzed by a machine-learning approach aiming to establish data infrastructure toward future aerogel studies. Data-evidenced critical discussion specifies a tendency in unreliable thermal conductivity data of these papers.
1 Introduction
1.1 Why Aerogels and What are the Issues?
Aerogels have been attracting increasing attention in many fields because they have the potential to significantly contribute to energy issues in the 21st century.[1] They can be key components for thermal superinsulators in residences, factories, transportation, and space architectures; high-surface-area electrodes for batteries/capacitors; low-dielectric-loss antennas for beyond 5G/6G technologies; purification/harvesting devices for water and food issues; scaffolds and drug carriers in biomedical fields; and CO2 adsorbents for carbon capture and storage technologies. Some of these potentials are indeed promising, while some of them are just an exaggeration for the impact of academic papers. Increasing attention to aerogels is causing an academic fever; in fact, aerogel was selected as a top ten emerging technology in chemistry by IUPAC (2022).[2] The number of papers on aerogels is exponentially increasing, as well as the number/scale of aerogel production in the industry.
Along with the academic fever, two serious issues are rising in the aerogel field: (i) “aerogel” hype and thoughtless uses of the word “aerogel” on anything porous. (ii) Unreliable experimental data, particularly thermal conductivities, not only in consumer advertising but also in academic papers.[3, 4] The aerogel field also cannot escape from some emerging issues in the whole academia along with the growing number of researchers, e.g., (iii) integrity issues, including too much exaggeration and disrespectfulness to previous studies.[5-7] We have a flood of aerogel papers with “excellent,” “high-performance,” “synergistic effect,” “rational design,” “bioinspired,” “multifunctional,” … in their titles. How many of the papers with these words are actually appropriate for the words? (iv) Too many review articles,[8, 9] containing a certain number of poor-quality reviews, like a copy and paste from a personal memorandum of journal clubs, lacking a balanced survey or bias-free overview. In fact, we have a total of 719 reviews, perspectives, and related types of papers whose titles contain “aerogel” up to 2024 (this will be discussed in Section 2.3).
To face these issues and contribute to the progress of aerogel research, we propose a new type of review article based on an exhaustive literature survey combined with a thoroughly data-driven analysis. The current review aims to provide an unbiased, comprehensive guide for aerogels with the following items: (i) an overview of the latest research status based on the exhaustive literature survey and statistical analysis on almost all of the existing “aerogel” papers; (ii) the first data infrastructure of process-property relation of polymer aerogels analyzed by machine-learning (ML) approach; and (iii) data-evidenced discussion specifying the items frequently connected to unreliable data toward what we need to keep the future community reliable. We also aim to push forward the idea of review articles to the next step; a “data-driven review” should be accompanied by systematic and exhaustive information curation and thoroughly data-evidenced discussion under critical viewpoints by active players in the field. Aerogels could be the first and suitable target of this attempt.
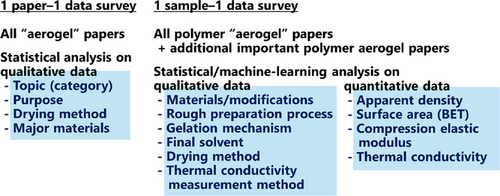
This review consists of three parts with two major surveys (Figure 1). First, the Introduction roughly summarizes aerogel definitions, processes, materials, applications, and industrial status based on statistics of the aerogel literature and industry. The second section focuses on deep statistical analysis using a one paper–one data dataset (17 429 papers manually checked one by one, ListA.pdf in the Supporting Information) from almost all the papers that contain “aerogel” in their titles. The third section focuses on polymer aerogels, in which unreliable data is causing a serious issue in the recent academic fever. Detailed and data-driven analysis is conducted using a one sample–one data dataset (16 559 samples, ListP.pdf in the Supporting Information) from all the polymer “aerogel” and some additional polymer aerogel papers.
1.2 What is Aerogel? A Brief History of Aerogel Definitions
According to the latest Handbook of Aerogels,[1] there is no unique definition of “aerogel” that covers all the reported aerogel materials, even though some groups tried to establish their own definitions from different viewpoints.[10-13] The current survey depicts the historical change of the objects that has been called “aerogel” (Figure 2).
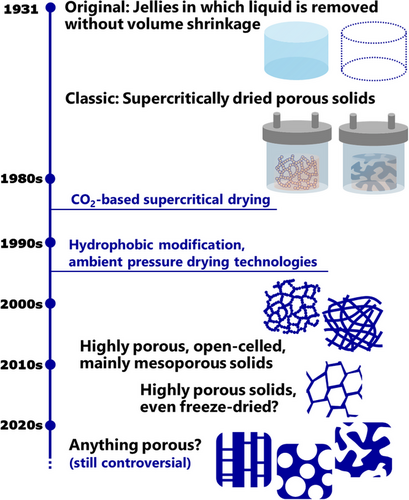
The first invention of aerogel (not the first usage of “aerogel,” see the inorganic section in 2.3) was reported in 1931 by Kistler.[14, 15] The original aerogel was a porous solid prepared by replacing liquid inside jelly with gas without or with minimum shrinkage of the jelly. The first method chosen was high-temperature supercritical drying ([SCD], Section 1.3 for details). After that, the definition of aerogel had been more or less “porous solid prepared by replacing the liquid inside gel with gas, particularly through SCD.” This classic definition had seemed to be widely accepted until the ∼2000s.
The solvents used in Kistler's report were alcohol and propane, which have relatively high critical temperatures. In 1980s,[16] supercritical CO2, which has a mild critical point (31 °C, 7.4 MPa), began to be used in aerogel production (supercritical CO2 itself had already been used in critical point drying for microscopy specimen preparation from the 1950s).[17] Supercritical alcohol-dried silica generally has hydrophobic surfaces with Si─OR (R from alcohol), while supercritical CO2-dried silica is hydrophilic with Si─OH.[18, 19] In the 1990s, hydrophobization and ambient pressure drying (APD) were developed to prepare low-density silica having aerogel-like microstructures (Sections 1.3 and 2.3 for details).[20-22] The definition of aerogel had kept in the range of “supercritically dried solid” for a few years after these inventions, while the ambient dried, low-density silica and resins were called “xerogel,” even xerogel has several different meanings (e.g., low/no porosity solid after evaporation of solvent in gel).[1] From the late 2000s to the 2010s, the aerogel definition had gradually expanded toward porous solids with specific microstructural features regardless of drying methods. A typical definition was highly porous (at least > 50%, typically > 90% in porosity), open-celled, and mainly mesoporous solid. These features were somehow essential to avoid overlapping with other porous materials; “highly porous” was needed to distinguish aerogels from mesoporous silicas, “open-celled” and “mainly mesoporous” were necessary to distinguish aerogels from foamed polymers, activated carbons, zeolites, metal organic frameworks (MOFs), and covalent organic frameworks (COFs). Despite some aerogels having a certain amount of micropores/macropores, the usage of “aerogel” had kept in the range of porous solids with the majority of pores in 10–100 nm until in the middle of the 2010s.
Freeze drying (FD) production of aerogel-related materials has been explored since the ∼1990s.[1] In the early stages, most of these macroporous solids had been called “cryogels” or “lyogels” (from lyophilization, another name for FD) at least until the ∼2000s. We note that cryogel has several meanings, including wet gel prepared through repeating freeze-thaw processes (cryogelation).[23] Along with an explosion of aerogel papers in the 2020s, a variety of porous solids have begun to be called aerogels in academic literature: from classic supercritically dried mesoporous aerogels, freeze-dried macroporous ones, assemblies of submillimeter-sized polymer fibers,[24, 25] delignified woods,[26] vapor-processed foams,[27, 28] to even foamed polymers/carbons.[29-31] Some of the above do not always respectfully consider how the word has been used in previous studies. It is still controversial whether such indiscriminate usages of “aerogel” are tolerated in the future academic community.
Another questionable definition of aerogel is that by IUPAC (2007):[32] “gel comprised of a microporous solid in which the dispersed phase is a gas. Note: Microporous silica, microporous glass, and zeolites are common examples of aerogels.” Almost none of today's aerogel researchers would agree with this microporous definition, and even almost none of the zeolite researchers would agree that zeolite is aerogel.
1.3 Drying Methods
The aerogel production generally consists of several sequential steps: gel formation, solvent exchange, and drying. There are a wide variety of gel formation routes, such as physical precipitation, aggregation, physical/chemical crosslinking, and polymerization, depending on the materials. Some processes do not need a clear gel formation step, i.e., the solution directly proceeds to the drying step. Solvent exchange is often required before drying. The final solvent depends on drying methods. Alcohols, acetone, and alkanes are typical for SCD and APD because of miscibility with high-pressure CO2 or low surface tensions. Water and t-butanol are typical for FD because of their decent melting points.
1.3.1 Supercritical Drying
SCD has been the gold standard for aerogel production. When a substance is placed at a temperature and pressure above its critical point, the substance becomes a supercritical fluid, which has no clear interfaces with liquid or gas. SCD can remove the liquid inside the wet gel without causing deformation or shrinkage because of the absence of liquid/gas interface tension.
There are roughly three types of SCD: (i) one solvent, high-temperature SCD using alcohols; (ii) one solvent, low-temperature SCD starting with liquid CO2; and (iii) two solvents, low-temperature SCD, namely the extraction of organic solvent with continuous CO2 flow. One-solvent SCDs (i) and (ii) use the liquid–supercritical fluid–gas route of one pure solvent (Figure 3a). This route can operate without continuous pumping, where the pressure can be controlled by temperature and an initial amount of the liquid. In some cases of alcohol-based one-solvent SCD, called rapid supercritical extraction,[33] you can skip a large part of the solvent exchange to reduce the operation time of aerogel production. The liquid CO2-started one-solvent SCD has the advantage of milder critical conditions of CO2; instead, the route requires time-consuming solvent exchange with liquid CO2 (typically several days).
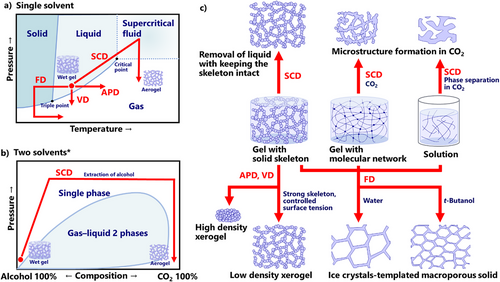
Two solvents SCD (iii) extracts organic solvents with a continuous flow of high-pressure CO2 (Figure 3b). Most organic solvents, e.g., alcohols, acetone, and alkanes, can form a single phase with CO2 under a certain pressure–temperature range. This route is much faster than liquid-CO2-based SCD because it does not need complete solvent exchange with liquid CO2 before increasing temperature. Instead, it requires a bit higher pressure to reach the single phase, continuous pumping of the inlet CO2 flow, and backpressure control of outlet flow.
As shown in Figure 3c, SCD generally does not affect the skeletal microstructure of wet gel because of the absence of surface tension. A recently reported exception is crosslinked chitosan gel,[34, 35] which does not have a clear solid structure before SCD and spontaneously forms a solid skeleton in supercritical CO2. In some cases, called phase inversion, supercritical or liquid CO2 acts as a trigger for phase separation to convert polymer solution into a solid skeleton.[36, 37]
1.3.2 Ambient Pressure Drying
APD does not avoid the liquid/gas interface (Figure 3a). Instead, it reduces the damage by capillary force by choosing low-surface-tension solvents with strong skeletal structures. APD was introduced in the aerogel production of hydrophobized silica in the 1990s.[20-22] Large and hydrophobic trimethylsilyl groups prevent hydrogen bonding between skeleton surfaces and cause spring back, i.e., shrinkage and recovery during drying, resulting in low-density aerogel-like structures. This technology was first reported in thin film silica, then bulk silica, and other oxide aerogels. Silicone and phenolic resin aerogels are also successful examples of APD (details are in the polymer and silicone sections of 2.3). As the surface tension of a cylindrical capillary is inversely proportional to the capillary diameter, skeletons with larger pores can be dried easier through APD without shrinkage. The APD process is sometimes modified with reduced pressure, which is classified as vacuum drying ([VD], Figure 3a) in the current survey.
1.3.3 Freeze Drying
FD is another method to avoid capillary force through the liquid–solid–gas route (Figure 3a). Freeze-dried solids normally have micrometer-sized macropores due to water ice crystals (bottom of Figure 3c). In general, faster freezing or a more rapid temperature jump results in smaller ice crystals and hence smaller pores. When other organic solvents, typically t-butanol, are used as solvents, the pore size can be decreased, e.g., translucent boehmite nanofiber cryogels.[38]
The FD process does not require a clear gelation step: just freezing and VD the solution. This is related to the physics of cryogelation.[23] The ice crystals formed at the early stage of freezing have a low solute/dispersant concentration because of a relatively higher melting point, resulting in a high concentration of solute/dispersant in the liquid remaining in gaps between the ice crystals (freezing point depression), which causes physical precipitation/aggregation and eventually forms a solid skeleton.
The current survey found some “aerogel” papers with other drying methods, e.g., spray drying (classified as “other” in this survey), and completely dried operation through the whole process, e.g., chemical vapor deposition (“dry process”).[27, 28]
1.4 Materials and Applications
Silica is the most popular and well-studied material of aerogels (Figure 4).[39, 40] Typical silica aerogels have highly porous (porosity > ∼90%), mainly mesoporous microstructure. They show a translucent appearance due to submicrometer-scale structural homogeneity; in other words, the spatial roughness of the refractive index is far smaller than the wavelength of visible light, resulting in a negligible Rayleigh scattering intensity. The apparent refractive index of silica aerogels can be controlled by changing apparent density, i.e., the volumetric ratio of air and silica, which is a suitable feature for experimental instruments in particle physics.
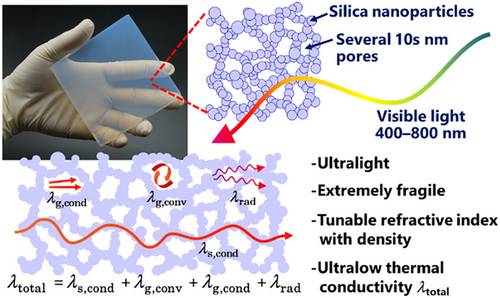
Homogeneous, highly porous structure of silica aerogels exhibits ultralow thermal conductivity (∼12 mW (m·K)−1 at the lowest)[39, 40] lower than that of static air (∼26 mW (m·K)−1).[41] The thermal conductivity of porous material can roughly be estimated by adding contributions from different modes (Figure 4, bottom).[42, 43] In the case of silica aerogels, solid skeleton conduction (λs,cond) is low because of high porosity, gas conduction (λg,cond) is low because of the small pore size close to the mean free path of N2 (so-called Knudsen effect), and gas convection (λs,conv) is almost negligible. This is the reason for the successful commercialization of aerogels as thermal superinsulators.
Figure 5 depicts typical aerogel materials (middle) and applications (top and bottom) reported in the literature, where the line width connecting materials and applications reflects the number of papers. Various materials of aerogels have been reported up to date: inorganic materials such as oxides, metals, semiconductors, layered hydroxides, carbides, nitrides, and fluorides; organic polymers from biopolymers to various synthetic polymers/supermolecules; carbon-based, graphene-based, silicone-based materials; and hybrids/composites of organic and inorganic components, including MOFs.
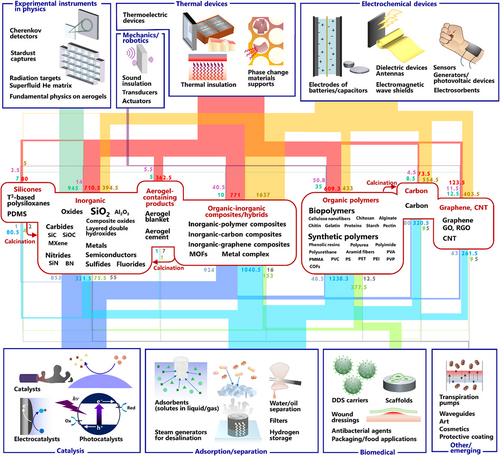
One classic and successful application of aerogels is instruments for experimental physics, such as Cherenkov detectors,[44-46] matrices for superfluid substance,[47-50] and stardust collectors.[51-53] Thermal insulation is another successful and widely commercialized application taking advantage of the ultralow thermal conductivity.[54-57] Not only the thermal insulation, but also the supports of phase change materials (PCMs) are emerging applications in thermal management technology.[58, 59]
Besides these successful applications, a variety of potential applications of aerogels have been proposed. Mechanical features of aerogels, e.g., sound/vibration insulation,[60, 61] ultrasonic transducers,[62] and actuators,[63] have long been investigated. Electrochemical devices, e.g., batteries/capacitors,[64-68] sensors,[69, 70] antennas and dielectric devices,[71] triboelectric/piezoelectric devices,[72] and photovoltaic devices,[73, 74] are popular targets in aerogel papers. Electromagnetic wave shield is a highly chosen application in very recent papers in the 2020s.[75-78] Catalysis,[79-82] electrocatalysis,[83, 84] and photocatalysis[85, 86] have long been major application targets based on their high surface areas. Adsorption/separation-related applications are major targets in recent papers, such as adsorbents for solution/gas pollutants,[87-89] separation of water/oils,[90-92] filters,[93] and hydrogen storage.[94] Recently, steam generation for purifying salt water is a frequently chosen application target.[95-97] In biomedical and food fields, monolithic aerogels for scaffolds of cells[98-100] and aerogel beads/microbeads for drug delivery carriers[101, 102] are the two most popular applications. Antibacterial/wound dressing sheets[103] and various packaging agents[104, 105] have also been proposed. Some of the above applications are really promising, and technology transfer to industry is under halfway or has already been completed, e.g., physics instruments, silica-based thermal superinsulation, carbon aerogels for battery electrodes, and oxide aerogels for catalysis, whereas some of them are just for eye-catching demonstration for the impact of papers lacking scientific insights or actual feasibility/efficiency consideration in the real world (detailed discussions are in each topic in 2.3).
The application fields are not limited to the above. A wide variety of applications have been proposed day after day, such as art,[106-109] cosmetics,[110] waveguides,[111] and Knudsen pumping,[112] a pump which can change a temperature gap to a pressure gap without an external power supply.
1.5 Industrial Status
Both SCD and APD have been chosen for aerogel production in industry.[113, 114] Large-scale production of pure silica aerogel monoliths is difficult because of their extreme fragility and the size restrictions of drying chambers. Most of the silica aerogel products are commercialized as composites, such as aerogel–non-woven fabric blankets, aerogel–polymer sheets/panels, aerogel cement, aerogel plaster, or as powder, granules, and granules-filled glazing. These products mainly focus on thermal superinsulation in residences, factories, transportation vehicles, apparel, and storage devices. One emerging product is flame-retardant silica aerogel composite panels for Li+ ion batteries, i.e., thermal runaway protection in electric vehicle batteries.[115, 116]
Despite the market size being one order smaller than that of silica, some non-silica aerogels have been commercialized.[113, 114] Carbon aerogels are commercially available mainly for battery applications. A thermal insulation panel of polyurethane aerogel was recently commercialized. Polyurea, polyurea-modified silica, polyimide, polyamide, and polybenzoxazine aerogels have also been commercialized in recent years. Silicone aerogel monoliths and granules made from methyltrimethoxysilane (MTMS) were commercialized from a start-up company. Some biopolymer aerogels are also commercially available, mainly as aerogel beads. In very recent years, large-scale production of polyimide aerogels has become one of the hottest targets in industry for the dielectric devices in the near future.
The major challenge in the commercialization of aerogels has always been the production cost,[117-119] including large initial costs for drying instruments as well as electricity costs. Some groups have tried to assess the economic/environmental impacts of aerogel products, where the results highly depend on chemicals, drying methods, and application areas.[120-122] Silica aerogels are excluded from the nanomaterial safety regulation under REACH of the EU.[123-125] Ecotoxicity and cytotoxicity studies show that aerogels are basically safe materials, whereas some researchers have pointed out the lack of investigation on some specific risks.
2 Exhaustive Survey on Aerogel Literature
2.1 Survey Conditions
The detailed survey condition is in Section S1 in the Supporting Information. In brief, we exhaustively searched the papers containing “aerogel” or “aero-gel” in their titles available up to 2024 on the websites of academic journal publishers. The papers are classified in nine categories by their topics: inorganic aerogels, organic polymer aerogels including polymers with minor carbon/graphene additives, organic-inorganic composite aerogels including carbon-inorganic composites and MOFs, pure carbon aerogels, graphene/CNT aerogels, silicone-based aerogels, physics topics on aerogels, application-focused papers using aerogel-containing products, and review articles. We extracted the drying method, purpose or target application, dimension/shape, main materials, and special treatments/notes/comments, if applicable, from each paper. When a paper contains more than one drying method/purpose, we counted it as equal contributions, e.g., one paper contains SCD and FD samples: 0.5 SCD and 0.5 FD.
2.2 Overview of Aerogels Research
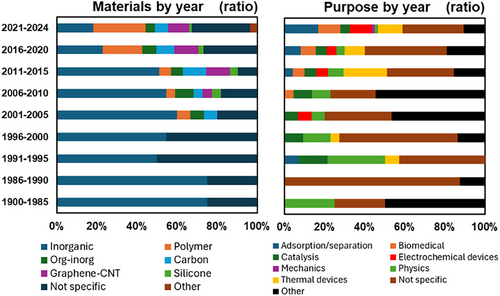
Figure 6 shows changes in the number and topics of aerogel papers by year. Until ∼2000, most of the papers on aerogels had focused on inorganic materials, mainly silica and several metal oxides, and their fundamental physics. After the 2010s, the papers on organic polymers and organic-inorganic composites have drastically increased and become predominant in the 2020s.
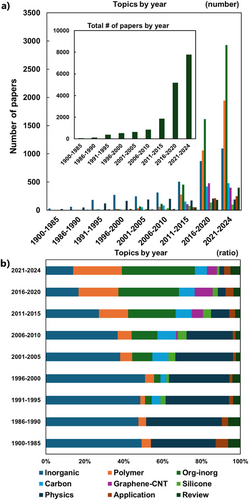
2.2.1 Drying Methods
Figure 7a shows the change in drying methods in the aerogel literature by year for all papers (left) and papers in respective topics (right). SCD had almost been the only method until ∼1995. After the invention of APD, it has gradually become an alternative route to aerogels. FD has drastically increased after the ∼2010s along with the explosion of polymer, organic-inorganic, and graphene aerogels, as most of which have been prepared by FD.
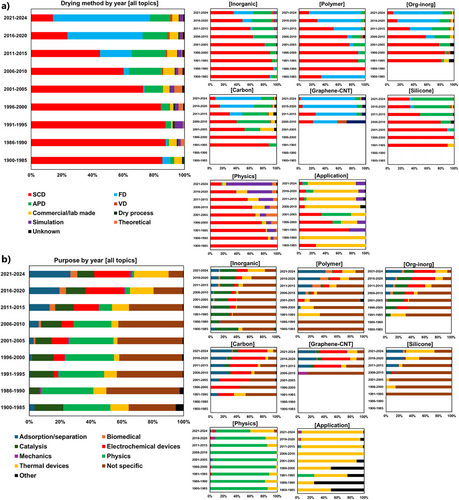
2.2.2 Purposes
Figure 7b shows the change in purposes, or application targets, of the aerogel papers. Classic aerogels until the ∼2000s were highly rated for instruments in experimental physics, such as Cherenkov detectors. Thermal insulation and catalysis have constantly been application targets from the early stage of studies up to now. Electrochemical devices and adsorption/separation have become major targets after the ∼2010s, but a certain amount of them do not consider actual feasibility in the real world, as will be discussed in the next section.
2.3 Topics of Aerogel Papers
This section focuses on the situation of each topic of the aerogel papers. Additional data are summarized in Figures S1–S8 in the Supporting Information.
2.3.1 Inorganic: The First Aerogel Was Metal?
The first report by Kistler (1931)[14, 15] on SCD of wet jellies, including silica, is well known to be the first invention of aerogel. But this is not the first appearance of “aerogel” in academic papers. At least Gibbs and Liander (1930)[126] used “aerogel” in the title, reporting vapor deposition of porous Ni in the context of “aerosol” accumulating into “aerogel.” We note that there may be earlier cases, as our online access to old papers would be limited.
Figure 8 represents examples of typical and latest inorganic aerogels. Until 1980s, major inorganic aerogels were silica, alumina, and other metal oxides.[127-130] After that, a variety of inorganic aerogels have been invented: metals,[10, 131-134] clays[135-137] (1980s, freeze-dried clays were already reported in 1950s), noble-metal-doped oxides,[138-141] BN[142-144] (1990s), fluorides,[145, 146] chalcogenides,[147-149] SiC[11, 150] (2000s), Si(B,C,N),[151-153] MXene[154-156] (2010s), and metallenes (2020s).[157] SCD is the major approach to inorganic aerogels because of the high brittleness of metal oxide aerogels. APD of hydrophobized silica appeared in the mainstream in the ∼2000s. FD of non-oxide ceramics, often prepared by pyrolysis of freeze-dried organic polymers, has become popular since ∼2015. Recently, 3D printing technology was established using SiO2-predispersed ink, which was followed by a variety of 3D-printed aerogels, including organic polymers.[158, 159]
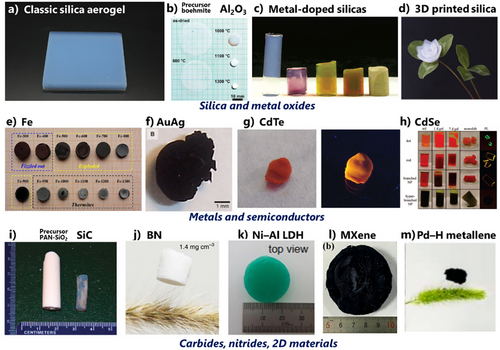
Classic applications of inorganic aerogels are instruments in physics, thermal insulation (mainly silica, on-site studies of silica aerogel-containing products are counted in the application topic), catalysis (metals and oxides, titania for photocatalysis), and electrochemical devices (metals and oxides for batteries, capacitors, and sensors). Recently, CO2 capture became a major potential application using amine-modified silica aerogels.[160]
2.3.2 Polymers: First Appeared in Kistler (1931), Exploded after 2010s
Some organic polymer aerogels, gelatin, agar, nitrocellulose, cellulose, and egg albumin were already reported in Kistler's first report.[14, 15] After that, polymer aerogels had rarely appeared until the ∼1990s, except for some fundamental investigations on polystyrene aerogels.[161-164] Phenolic families (e.g., resorcinol–formaldehyde resins) appeared in the 1980s, which continued to the invention of carbon aerogels.[165-168] In contrast to inorganic aerogels, structural homogeneity of polymer aerogels, i.e., making transparent/translucent polymer aerogels, is hard to control. There have been a few cases reporting transparent polymer aerogels.[169-184] Successful cases are cellulose and its nanofibers,[169-171] chemically crosslinked and physically precipitated chitosans,[172-177] polyimide,[178, 179] and melamine–formaldehyde[180] (Figure 9, top).
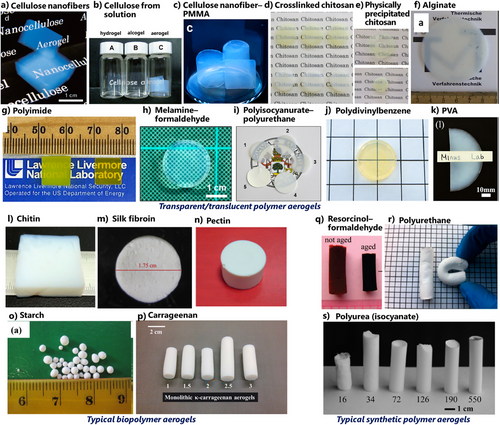
Biopolymer aerogels (Figure 9, bottom left) have been in hype since the ∼2010s.[185, 186] Cellulose and its nanofibrous forms,[169-171, 187, 188] chitin,[189, 190] chitosan,[172-177, 191] alginate,[192, 193] pectin,[194] carrageenan,[195, 196] agar,[197] starch,[198, 199] silk fibroin,[200] and gelatin/collagen[201, 202] are frequently chosen biopolymers for aerogels. Some researchers have suggested potentials of SCD-based biopolymer aerogels for thermal insulation[203, 204] and drug carriers[101, 102] and FD-based ones for scaffolds,[98-100] some of which look promising so far. Recently, a continuous production of biopolymer aerogel beads was reported.[205] APD effectively works on some synthetic resins, e.g., resorcinol–formaldehyde aerogels, while APD of pure biopolymer aerogels is still challenging. Some attempts were reported on cellulose but resulted in high density.[206-208] A tremendous number of papers in the 2020s have reported aerogels through simple FD of aqueous biopolymer solution/suspension, most of which have been suggested as pollutant adsorbents. From a critical viewpoint, the majority of these FD-based adsorbent papers lack scientific insights or actual demands in the real world.
Synthetic polymers (Figure 9, bottom right), polyurea,[209, 210] polyurethane,[211] polybenzoxazine,[212, 213] ring-opening polymers such as polydicyclopentadiene,[214, 215] polyamide/polyimide and their fibers,[216-218] keep expanding their fields mainly in thermal insulation and adsorption. Conductive polymers, such as polypyrrole, polyaniline, and PEDOT:PSS, have been investigated for electrochemical applications.[219] Polyimide aerogel has become a hot material for dielectronic and electromagnetic devices in recent years. COF aerogels have also become popular.[220] Very recently, recyclable polymer aerogels have attracted much attention in the context of the worldwide issue of waste plastics.[221-223]
2.3.3 Org-Inorg: Polymer-Reinforced Silica to Diverse Composites
Silica aerogels hybridized with organic polymers on their surfaces, sometimes called X-aerogels, appeared in the 1990s.[224-226] They proved the potential of oxides–organic polymer composites, particularly in mechanical flexibility improvement. A variety of configurations of composite aerogels have been reported to date: from hybrids at the molecular level, composites in the nanoscale, micrometer scale, to simple mixtures over the submillimeter scale. Some of them are transparent, i.e., less light scattering due to structural homogeneity in nanoscale, e.g., polymer-modified silicas,[224-228] polysiloxanes composited with cellulose[229, 230] and amyloid nanofibers,[231] and chitosan aerogels compositing with luminescent nanoparticles.[232] Recently, a general route to transparent composite aerogels of MTMS-based polysiloxane and water-soluble biopolymers was reported without introducing covalent bonds between the two components (Figure 10, top).[233]
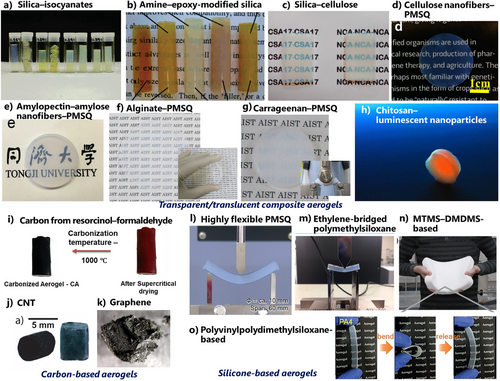
Metal-supporting polymers/carbon/graphene aerogels became popular in the 2010s for various catalysis and electrocatalysis applications.[234, 235] After ∼2020, papers on FD-based mixture aerogels made of oxides/metals + biopolymers/carbon/graphene have drastically increased for electrochemical applications, particularly for batteries/capacitors,[64-68] sensors,[69, 70] and electromagnetic wave shield.[75-78] Critically speaking, most of these application demonstrations in recent papers would be just for increasing the impact of the papers with eye-catching figures, lacking scientific insights or feasibility in the real world. It is also questionable that we should call these FD-based composites “aerogels,” where a certain amount of similar FD materials are not called aerogels.
2.3.4 Carbon: Conductive Mesoporous Materials
Carbon aerogels were first prepared from resorcinol–formaldehyde and related phenolic resin aerogels through pyrolysis in a reducing atmosphere (Figure 10, bottom left).[236-239] Carbon aerogels usually have electric conductivity and unique mesoporosity with high surface areas suitable for battery electrode applications. Incorporation of KOH before calcination and/or calcination under CO2 atmosphere, called “activation,” is often adopted to improve meso/microporosity.
The precursor resin aerogels were originally prepared by SCD.[237] APD became popular in the ∼2000s, and then it was overcome by FD in the ∼2010s. Biopolymer-based carbon aerogels, namely freeze-dried and pyrolyzed biopolymer solution/hydrogels, have become a popular route to carbon aerogels since the 2010s.[240] These materials lack mesoporosity because of FD.
2.3.5 Graphene-CNT: Diverse Usages of “Graphene” and “Aerogel”
Graphene aerogels appeared in the 2000s (Figure 10, bottom left),[241-243] to be accurate, some people started to call macroporous graphene/graphite solids “aerogels.” The word “graphene” also contains chemical variations: pristine monolayer graphene, multilayered graphene, graphene oxides, and reduced graphene oxides; some of them are actually graphite. In spite of the same chemical component, graphene aerogels are totally different from carbon aerogels, which are typically prepared by pyrolysis of organic resin aerogels. Graphene aerogels are typically prepared by hydrothermal aggregation of graphene whiskers in suspension, followed by FD.[241-243] They have ice-derived cellular macroporous structures with relatively lower densities than those of carbon aerogels. Recently, papers on pure graphene aerogels have decreased, while those on graphene-containing composite aerogels have increased (counted mainly in the org-inorg topic).
The first CNT aerogel was reported in 2007 (Figure 10, bottom left).[244, 245] CNT is not popular as a main component of monolithic aerogels but as a filler of composite aerogels for increasing electric/thermal conductivity. Pure CNT coatings or sheets grown through all-dry processes, typically through chemical vapor deposition, are also called “aerogels” in some papers.[246]
2.3.6 Silicones: A Key to APD Manufacturing
The fundamental chemistry of (RO)n─Si─R’4−n ingredients was well studied in the 1990s.[247-249] APD of silicone-based aerogels, xerogels at that time, has been extensively reported after transparent MTMS-based xerogels in 2007.[250] MTMS itself is more expensive than pure silica ingredients (water glass, TEOS), while it does not need additional hydrophobization because of the original methyl groups.
In the 2010–2020s, a variety of silicone/polysiloxane aerogels,[251-255] such as (SiO)3─Si─R’ (e.g., MTMS-derived polymethylsilsesquioxane), (SiO)2─Si─R’2 (e.g., polydimethylsiloxane), and alkyl chain-hybrid polysiloxanes, have been developed, mainly focusing on improving mechanical flexibility compared to pure silica aerogels (Figure 10 bottom right). In particular, the strong compression spring-back nature of alkylsilsesquioxane skeletons is sometimes suitable for APD preparation. This nature is also effective in some of the MTMS–biopolymer nanofiber composites.[231] Very recently, unusually flexible and highly transparent MTMS-based aerogel/xerogel have been archived through precise control of skeletal microstructure.[256, 257]
To eliminate the arbitrariness of the survey, we only counted the papers that had “aerogel” in their titles. We note that this survey underestimates the number of APD silicone aerogels because the early papers tended to use “xerogel” instead of aerogel.
2.3.7 Physics: Giant Instruments to Computer Simulation
Aerogels, mainly silica, had been paid much attention as a unique matrix material for particle physics, cosmology, and condensed matter physics: Cherenkov detectors from the 1970s,[44-46] matrices of superfluid substances (silica, alumina, Nafen) from the 1980s,[47-50] and stardust collectors from the 1990s.[51-53] Since most of the above cases require a homogeneous monolithic structure with a precisely controlled refractive index, SCD is necessary for these applications.[258, 259] Along with these physical instruments, physical characteristics of aerogels have been well-studied by means of light/neutron scattering and acoustic methods.[260-262]
In the 2010s, papers for fundamental physics gradually decreased after technology transfer to respective fields, while simulation studies appeared in the mainstream along with progress in computer capability.[263] Many have focused on structure modeling, mechanics, heat transfer, and the microstructure formation process, although there would still be a certain gap from the actual phenomena.
2.3.8 Application: On-Site Evaluation, Simulation, Making Cement
Commercially available silica aerogels and aerogel-containing products have been expanding in recent years.[113, 114] These products have been used for on-site studies for thermal management of residences from the aspects of energy saving, cost estimation, and long-term durability. Such on-site demonstration and simulation studies have increased since the 2010s.[264-266] Extremely low density of silica aerogels and their unique rheological behavior in colloids/pastes make their handling difficult in industrial processes. There is still a large room for developing new methodologies to cement/plasters/panels containing homogeneously dispersed aerogel particles at a high concentration.[267-269] Very recently, a translucent aerogel brick was invented and proved its high insulation performance as well as good visual design.[270]
2.3.9 Reviews
Figure 11 summarizes the change in materials and application targets of aerogel review articles, including perspectives, editorials, and related types of papers, by year. The change in materials is roughly consistent with the ratio in the non-review papers (Figure 6). A small exception is a recent flood of biopolymer aerogel reviews; however, most of which are not very high in quality as they simply arrange the personal memorandum of picked-up papers. The number of reviews has drastically increased compared to those in the 1990s–2010s (the far-right dark green column in Figure 6b). A similar tendency of increasing reviews has been reported in other academic fields.[8, 9] From the longer viewpoint, the recent ratio of reviews/total papers is almost the same as that in 1900–1990, i.e., the ratio has kept roughly constant in the past 100 years.
2.4 Topics–Drying Methods–Purposes Analysis
To visualize the relation between topics, drying methods, and purposes of the papers, we carried out correspondence analysis (Figure 12). This analysis is a kind of principal component analysis focusing on the correlation between two qualitative items. The vertical and horizontal axes in Figure 12 do not have specific meanings; they are the first and second principal components. Items that are plotted close to each other in correspondence maps have a high correlation coefficient, meaning that the items often appear together in the same literature in our literature dataset.
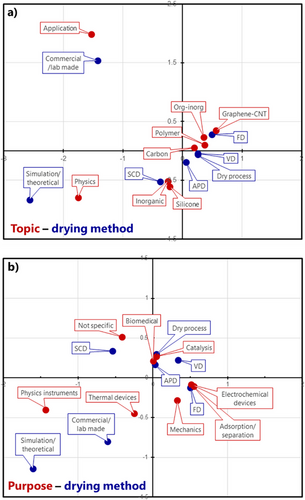
We conducted two analyses: relations between topic–drying method (Figure 12a) and purpose–drying method (Figure 12b). The “review” in the topic, “unknown/other” in the drying method, and “others” in purpose are omitted from the analysis to decrease the uncertainty. We note that the analysis contains a certain uncertainty, particularly on the items with small sample numbers (e.g., “dry process” in the drying method).
2.4.1 Topic–Drying Method (Figure 12a)
SCD and APD are the first and second closest drying methods, respectively, to inorganic and silicone. This means these materials are often prepared by SCD and APD. FD is the closest one to org-inorg and graphene-CNT. Carbon and polymer appear in the middle of FD, VD, and APD, indicating that these materials are prepared by various methods. Application and physics are far from other topics and close to commercial and simulation, respectively, which are reasonable.
2.4.2 Purpose–Drying Method (Figure 12b)
Adsorption (adsorbents, separation, steam generation) and electrochemical devices (batteries, capacitors, sensors, electromagnetic wave shields) are stuck to FD, meaning these purposes are studied with freeze-dried macroporous solids. Thermal devices, most of which are thermal insulation and PCM supports, are in the middle of SCD, FD, VD, and commercial, meaning that various types of porous structures have been investigated for thermal purposes. Catalysis is in the middle of APD, VD, SCD, and FD. This could be attributed to a variety of catalytic materials, from inorganic metals/oxides to metals/oxides-supporting carbon composite aerogels. Biomedical is also in the middle of APD, VD, SCD, and FD. This means two major applications, SCD beads for drug delivery and FD/APD scaffolds for cell cultures, but also contains a large uncertainty due to the relatively small number of papers. Not specific is close to SCD, meaning that the fundamental chemistry of aerogels, including classic aerogel synthesis research without a specific purpose, has been studied mainly with SCD samples.
2.5 Short Summary: What is Aerogel Again?
-
The mainstream of aerogels research had been SCD of inorganic materials until ∼2010. Organic polymers and composites have exploded after ∼2010. At the same time, a certain number of researchers have started to call FD-based porous materials “aerogels.”
-
Major applications of aerogels had been limited in experimental physics, thermal insulation, and catalysis until the ∼2000s. The application fields have been expanding after the ∼2000s. Adsorption and electromagnetic devices have become major targets of aerogel papers, but most of them have a certain distance from the actual uses in the real world.
-
There are some biases, or clusters, in aerogel studies. SCD is often selected for inorganic, silicone, and a part of polymer aerogels for physics, thermal, and drug delivery applications. APD is selected for inorganic, silicone, and carbon-based aerogels for catalysis, biomedical, and thermal applications. FD is chosen for polymer, graphene-based, and composites aerogels for adsorption and electrochemical devices. On-site evaluation of thermal insulation is often conducted with commercial aerogel-containing products.
From item (3), we can point out that there are different clusters of researchers. One group of researchers tends to use the classic definition of aerogels, supercritically dried, or a definition based on a highly porous mesoporous structure, and mainly focuses on thermal, catalytic, biomedical applications, and the fundamental physicochemistry of aerogels. Another group uses commercial aerogel products for on-site thermal insulation studies where the drying method does not matter. Another group thinks almost all of the porous materials, including FD ones, can be called aerogels, while most of their papers often claim potential applications like adsorbents and electromagnetic wave shields, but without facing actual uses in the real world. If we are allowed to use a strong expression, a certain part of the last group is “papers for the number of papers.” This review does not accuse these papers, as they are an inevitable piece of academic activity in the real world.
-
The flood of “freeze-dried aerogels” does not mean we have to include all FD materials into the definition of aerogels. In fact, a certain number of aerogel researchers would deny this idea, or some of them even do not know the flood situation as they live in different research clusters.
-
On the other hand, we cannot simply say, “Freeze-dried solids are not aerogels,” after the tremendous number of papers. The “highly porous, mesoporous, open-celled solids” definition of aerogels would hardly be a unique definition.
Thus, as the Handbook of Aerogels mentions,[1] there is no unique definition of aerogels, and even some of the researchers do not think we need, or should make, a unique definition of aerogels. At least this review proposes we should keep watching the situation with data and occasionally make an action for too much misleading usages of “aerogels” that would disturb healthy activities in academic/industrial communities.
3 Data-Driven Analysis on Polymeric Aerogels
3.1 Data Curation Conditions
With continuously increasing attention to biopolymers as sustainable alternatives in many fields, polymer aerogels are in a hot fever, as evidenced by the increasing number of papers (Figure 6). Unfortunately, such a fever also attracts low-quality studies with unreliable data. In this section, we focus on quantitative data on polymer aerogels to construct a foundation of data infrastructure for future studies.
The detailed survey conditions are in Section S2 in the Supporting Information. The curation condition is the same with one paper–one data analysis, except for the following additional items. We extracted additional qualitative data of skeletal materials, modifications, rough preparation processes, gelation mechanisms, the final solvent before drying, a rough type of SEM microstructure, and a thermal conductivity measurement method, if applicable, from each sample. Quantitative data of apparent density, porosity, Brunauer–Emmett–Teller (BET) surface area, compression modulus, and thermal conductivity were also extracted as long as possible.
3.2 Density Dependence of Physicochemical Properties: Data Visualization
Figure 13 shows porosity, BET surface area, compression elastic modulus, and thermal conductivity as functions of apparent density, classified by drying methods (Figure 13a), gelation mechanisms (Figure 13b), and types of skeletal materials (only for aerogels made of single material, Figure 13c). The largest difference between SCD and FD aerogels is apparent density. FD samples contain a large number of low-density ones, particularly in the < ∼0.1 g cm−3 region. This would reflect the fact that FD does not always need gelation; they can just freeze and dry a polymer solution or colloid. In contrast, SCD normally needs clear gelation before solvent exchange, resulting in relatively high-density samples.
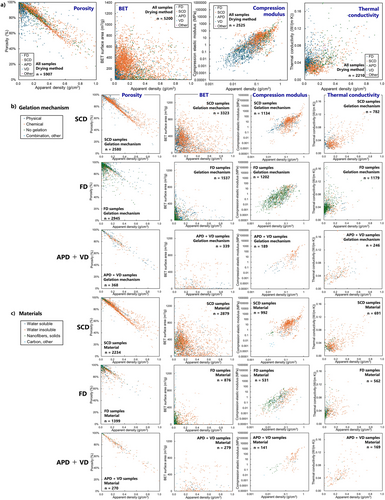
3.2.1 Porosity
Porosity linearly decreases with density, which is reasonable, regardless of drying methods, materials, and gelation mechanisms. The plots contain some exceptions from the linear relation, too low/high porosity, which would be due to experimental errors and/or inaccurate measurements.
3.2.2 Surface Area
Judging from the plot, BET surface areas of SCD aerogels are 10–100 times larger than those of FD aerogels. This would be attributed to less influence on microstructures by SCD in contrast to the micrometer-sized ice structure of FD. Among the SCD samples (the first row in Figure 13b), physical gelation tends to result in lower surface areas. This is reasonable considering a general tendency of gel and structure formations (Figure 14).[35, 174]

One feature of polymer aerogels is the distinguishment of gel formation and solid skeleton formation. In the cases of inorganic aerogels, gelation directly means formation of a solid skeleton, which is not always true in polymer aerogels. Some polymers form a wet gel of a crosslinked molecular network holding solvent inside the network without a clear solid phase. This network often converts into a solid skeleton after being exposed to antisolvent.[35, 174] Thus, general routes of polymer aerogels starting with a solution can be described with three events (Figure 14): gel formation, solid skeleton formation, and solvent removal. The timing of solid skeleton formation is the key to structural homogeneity. When the gel and solid skeleton form at the same time, the microstructure tends to become larger than the submicrometer scale and have less surface area, which is most often in the case of physical gelation.
Some BET data exceed ∼1000 m2 g−1, which is unbelievable as a polymer aerogel. These values would not be attributed to mesoporous aerogel skeletons but to microporous components, such as COFs. We note that BET measurement itself has several issues; some low-quality papers do not conduct the measurement/calculation properly, e.g., over several 100 m2 g−1 in simple water-freeze-dried polymer solutions.
3.2.3 Compression Modulus
The modulus linearly increases with density in log–log scale regardless of drying methods, materials, and gelation mechanisms, which is reasonable. SCD aerogels have a relatively higher modulus because of a relatively large sample number in higher density. From other viewpoints, no clear tendency is recognized in the scatter plots.
3.3 Thermal Conductivity and Unreliable Data Issue
Thermal conductivity measurement methods are divided into two types: steady state and transient methods. The former contains guarded hot plate and heat flow meter methods; most of them require a large sample and take a long time for each measurement, but with high accuracy. The guarded hot plate does not need any reference samples to guarantee the absolute conductivity values. The transient methods contain hot disk, hot wire, laser flash, some needle probes, and three-omega methods. Most of them are fast and easy but cannot guarantee absolute conductivity values in low conductivity regions. Figure 15a,b shows thermal conductivity data by means of different measurement methods and from different drying methods. The data are distributed roughly above a U-shaped area as a function of density, while we have a lot of unusually low conductivity data in the low-density region, particularly in < ∼0.1 g cm−3.
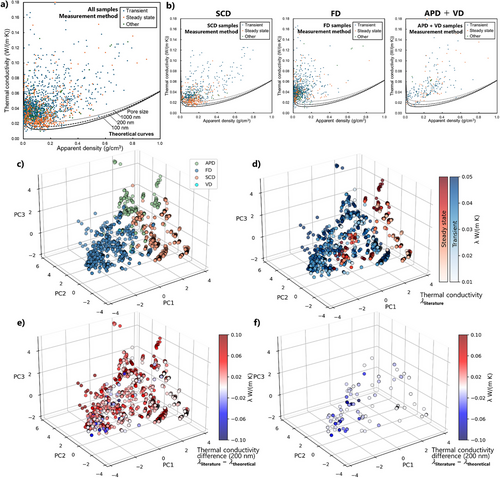
As discussed in Section 1.4, the thermal conductivity of porous materials is roughly estimated as the sum of the contributions of different modes, which can be calculated from the apparent density and pore size based on the assumption of perfectly homogeneous porous structures. The estimation gives a lower theoretical limit of the conductivity representing the U-shape as a function of density. We calculated the limit using Forest et al.[42] with different pore sizes, 100 (typical in most of transparent SCD aerogels), 200, and 1000 nm (most of FD aerogels), and plotted it together with the literature data in Figure 15a,b. Malfait et al. recently reported a similar estimation based on 519 data from aerogel papers regardless of organic or inorganic aerogels.[3] They found that FD samples and transient methods often exceed the lower limit of theoretical conductivity, meaning physically impossible, unreliable data. Our current analysis based on 2210 plots only from polymer aerogels shows the same tendency. In particular, a lot of FD samples (the middle of Figure 15b) exceed the limit of 1000 nm pore size. Considering the majority of FD samples have > 1000 nm pores due to ice crystals, a certain number of FD data are highly likely unreliable. These unreliable data were measured by both transient and steady-state methods. Besides, most of the obviously strange data, like ∼0.01 W (m·K)−1 at 0.01 g cm−3 density, were measured by transient methods.
3.3.1 Cluster Analysis
We conducted cluster analysis on the above thermal conductivity data (Figure 15c,d, details are in Section S3 in the Supporting Information). In the 3D map (the axes are first, second, and third principal components without specific meanings), the data are roughly classified into three clusters (see Figure S9a for details), mainly reflecting the drying method. Clusters I, II, and III mainly consist of APD, FD + a small number of VD, and SCD samples, respectively. The majority of Cluster II was measured by transient methods. Clusters I and III were measured using both transient and steady-state methods.
Instead of thermal conductivity itself, we also used the thermal conductivity difference between literature values and theoretical limits from the curve of 200 nm pore size (λliterature − λtheoretical; other pore sizes are in Figure S9c). Negative values mean exceeding the theoretical limits (bluish plots in Figure 15e; only the negative values are picked up in Figure 15f). Judging from this plot, bluish plots are concentrated in Cluster II, which mainly consists of FD samples measured by transient methods, meaning these conditions tend to result in unreliable data. Clusters I and III also contain a small number of bluish plots. Cluster III, mainly SCD samples, contains some white plots (close to the theoretical limit), but they do not drastically exceed the limit. We note that the theoretical curves in the current analysis are just a case of one simple model. Different models could vary the results. We also note that “within the theoretical limit” does not always mean appropriately measured data, which cannot be detected in this analysis.
Both Malfait et al.[3] and the current analysis show FD samples and transient methods are occasionally not reliable in the low thermal conductivity region. We have to be careful about the interpretation of this tendency, as correlation does not imply causation. Perhaps there would be other factors, e.g., some of the research projects that casually choose easy transient methods tend to disregard data accuracy in the first place. Also, steady-state methods do not always guarantee accuracy. In fact, in our previous study, the conductivity value began to lose its accuracy when the sample was thinner than ∼5 mm in the steady-state heat flow method.[271]
In the ideal world, all researchers should use the guarded hot plate method with 30 × 30 cm2 × 2 cm thick square samples to guarantee the absolute conductivity value. This is impossible in the actual world, particularly because of the sample size. The data do not say all the transient methods are unreliable; proper measurement with proper samples in the proper applicable range can bring appropriate values. Thus, we should keep discussion on standardization, or making a guide, of measurement in the low conductivity region. Establishing standard samples in the low conductivity region using aerogels (≤ ∼20 mW (m·K)−1 range. We have a ∼33 mW (m·K)−1 standard glass fiber board from NIST, which is one of the widely applicable low conductivity standards)[272] is one idea, but this is not easy considering the preparation, inspection, and handling of high-quality monolithic aerogels. Another idea is a clear distinguishment between guaranteed measurements and unguaranteed easy-to-do measurements, such as a rough screening of insulation performance with infrared camera imaging. The problem is that some researchers often pretend as if their unguaranteed conductivity measurements are accurate. In that case, they should clearly state that the methods are unguaranteed, which is nothing to be ashamed of, in their papers. Then, if they find some samples that look promising through the unguaranteed screening, they can proceed to the next step with a large sample and reliable steady-state methods.
For now, we have to encourage every researcher to recognize that accurate thermal conductivity measurement is always difficult and to take extra care with data inaccuracy. At least we should not choose transient methods, particularly in the low conductivity region or with FD samples, when we need accurate values.
3.4 Machine-Learning Analysis
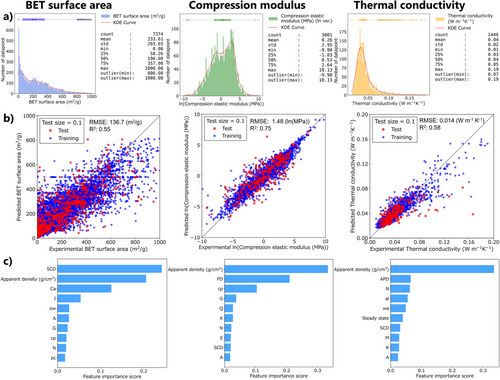
ML analysis was conducted on three targets: BET surface area, compression modulus (in log scale), and thermal conductivity. Details in data preprocessing, preliminary model investigation, and calculation conditions are in Section S4 in the Supporting Information. First, we evaluated the prediction accuracy for each of the three targets using seven regression-based ML models: least absolute shrinkage and selection operator (Lasso) regression,[273] ridge regression (Ridge),[274] Gaussian process regression (GPR),[275] support vector regression (SVR),[276] random forest regression (RFR),[277] extra tree regression (ETR),[278] and gradient boosting regression with Light Gradient Boosting Machine (LightGBM).[279] The former 6 are implemented in the scikit-learn package (ver. 1.5.2),[280] and LightGBM is used for the LightGBM package (ver. 4.5.0). Among these models, the decision tree-based approach (RFR, ETR, and LightGBM) performed relatively well, and in terms of the coefficient of determination (R2), the ETR achieved the highest prediction accuracy (Figures S10–S12, Table S4), which we used in the following investigation.
Figure 16a–c shows overviews of the target data distributions, 90%/10% training-test error plots, and feature importance plots for the 10 input items that contributed the most to predicting each target. A key advantage of the decision tree-based model is its relatively high interpretability; the impact of each input feature on the target properties can be quantified and ranked in the feature importance profiles (Figure 16c). Among all the targets, apparent density has a predominant influence—a finding that, while unsurprising, is consistent with the scatter plots in Figure 13a. Depending on the target, other important decisive features arise from drying methods, modifications, gelation mechanisms, and material categories.
To further investigate the results, we employed SHapley Additive exPlanations (SHAP, ver. 0.42.1)[281, 282] to interpret the importance of each feature and its contribution to the predicted values—that is, the rationale behind the ML model's predictions. SHAP summary plots (Figure 17a,c,e) shows not only the order of feature importance but also its direction of influence; the horizontal axis represents the impact of the input item on target values (negative to positive from left to right), and red/blue means small/large or absence/presence of input items.
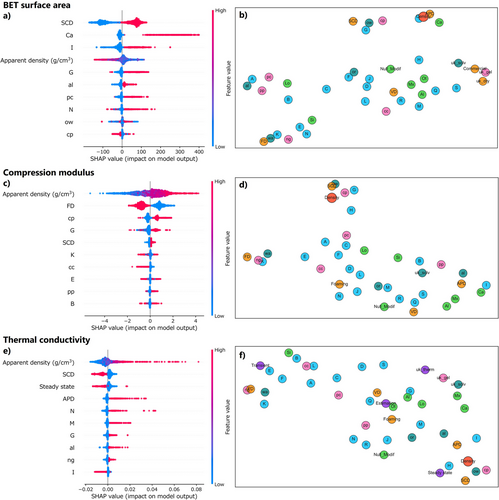
For example, when BET surface area is the target (Figure 17a), SCD and modification_Ca (calcination) appear in red in the region where the SHAP value (horizontal axis) is positive, indicating that both SCD and calcination strongly increase the BET surface area. The presence of material_category_I (phenolic resins) also has a positive influence, which may be attributed to the homogeneous nanoparticulate microstructures of phenolic aerogels and/or their frequent association with subsequent calcination (as shown in the correlation map of the input features in Figure S13). Apparent density generally has a negative effect, meaning that lower density tends to result in higher surface area. However, the graph shows a gradient from blue to red with some irregular points, indicating considerable variability in its effect.
For compression modulus (Figure 17c), density, gelation_mechanism_cp (chemical polymerization), material_category_G (polyamide/imide/imine), and SCD all exert positive influences, whereas FD shows a negative effect. These trends can roughly be explained by differences in density and the use of polyimide-related materials; higher density corresponds to higher modulus, SCD tends to yield relatively high-density samples, and FD often results in low-density samples. Polyimide-based materials are well regarded for their high modulus and are frequently prepared via chemical polymerization. The above relations are also supported by the correlation between respective input features; density has a decent correlation with SCD (positive) and FD (negative), and chemical polymerization and polyamide/imide/imine also have a positive correlation (Figure S14).
For thermal conductivity (Figure 17e), density generally exerts a positive effect with some variability, SCD exhibits a clear negative impact, and APD shows a clearly positive influence. These results are consistent with common trends in thermal conductivity; apparent density strongly affects conductivity but not strictly in a linear manner; SCD promotes mesoscale homogeneous structures that lower thermal conductivity; and APD tends to increase the density. Steady-state methods also have a negative influence, which could be attributed to a loose correlation with SCD (Figure S15); researchers who often use SCD for aerogel production tend to use steady-state methods.
Additionally, we visualized the high-dimensional input feature vectors on a two-dimensional plane using Uniform Manifold Approximation and Projection (UMAP, ver. 0.5.7)[283-285] UMAP is a dimensionality reduction method that projects input features from the original high-dimensional feature space, i.e., many variables from many input items, onto a two-dimensional plane where the features that are close in the original space remain proximate. In the UMAP of BET surface area (Figure 17b), the features with high importance scores and strong predictive influence, such as SCD, modification_Ca (calcination), material_category_I (phenolic resins), and density, cluster in the upper region. This clustering suggests that these features are relatively close in the original high-dimensional space, indicating a high degree of similarity among them. Similarly, that for the thermal conductivity (Figure 17f) shows that key features, including density, SCD, steady-state methods, and APD, aggregate in the lower right. On the other hand, the UMAP for the compression modulus (Figure 17d) reveals that the most important feature, density, is positioned in the upper central region, while the second most important feature, FD, is located at the left edge, meaning that both items have decently independent contributions to the predictive accuracy of the model.
Finally, we have listed samples with outlier properties, i.e., the samples having large deviations between actual and predicted values, using leave-one-out cross-validation (Tables S5–S7, Figures S16–S18). The outlier plots in the BET surface area regression contain exceptionally high surface areas from the samples prepared by both SCD and FD. The main reason is the small number of data with high BET surface areas, but it also suggests that the factors that give high BET surface areas may not be sufficiently provided as input features. Those for compressive modulus tend to show low moduli, which could be attributed to experimental errors such as surface roughness of the samples. The thermal conductivity contains mainly high conductivity data compared to expected values, most of which were measured using transient methods. It should be noted that these outlier plots are not always indicative of the reliability of the data.
The ML models constructed for this review all use category variables, i.e., 0 or 1 for absence or presence, as input features, except for density. Such models are relatively easy to construct and suitable for capturing general trends in the dataset, but it is difficult to say that they can learn physicochemical properties of raw materials or preparation conditions. If it is possible to express these properties as appropriate unique feature vectors using “feature engineering,”[286] it would not only improve the prediction accuracy of the model toward future predictive studies for desired properties but also give us some information about the data reliability.
3.5 Short Summary: Toward Aerogel Data Infrastructure
In the data visualization, most of the data are reasonably distributed, being consistent with researchers’ traditional intuitions; SCD samples have high surface areas, compression modulus is roughly proportional to density in log scale, and thermal conductivity roughly shows a U-shape with density. The ML regression analysis on these targets is also successful as the feature importance and SHAP analysis are reasonable along with conventional knowledge. Besides, some strange data are also specified, e.g., unusually high surface area in FD samples and unreliable low thermal conductivity in FD samples or by transient methods; the latter is well clarified in the cluster analysis.
The analysis verifies that our dataset would be the first data infrastructure for future polymer aerogel studies. At the same time, we have faced several issues with this approach: (i) Quality and bias of data. The current data come from papers with a wide range of research quality. There must also be a bias; good data appears in publication more often than bad data. In some cases of data-driven catalyst research, researchers construct their own high-throughput instruments for a continuous synthesis–evaluation system to keep the data accuracy and precision at a fixed level.[287, 288] In aerogel studies, such an approach is difficult since aerogel synthesis normally requires long solvent exchange and drying processes, aerogel characterization needs some day-long measurements, and aerogel properties frequently depend on sample sizes. (ii) Detection of unreliable data. We have spotted some unreliable data, but they cannot be detected without a conventional knowledge-based classification using non-data items, e.g., the theoretical limit of thermal conductivity. Data themselves do not tell us which parts of them are unreliable; more precisely, data can spot outlier plots but do not tell us about their reliability. Perhaps we would be able to enrich metadata information and take it into the analysis, such as when, where, by whom, and with which instruments the data was collected. These metadata could bring a little improvement in data reliability evaluation, but this is not the best idea from a feasibility and also poses a potential risk of raising a political correctness issue. At least in the context of academic fever in the 2020s, we have to keep the fact in mind that there are inevitably some unreliable data in the literature. These items remind us of a trivial but important rule: data must be combined with accumulated knowledge in the research field.
4 Summary and Outlook
The current review has provided a thoroughly data-driven overview of aerogels based on an exhaustive “aerogel” literature survey up to 2024. A systematic overview of the current research status shows several characteristic items: (i) Aerogel materials and applications have become wider and wider in this decade. They could be a key contribution to energy issues in the 21st century, such as superinsulation in residences and vehicles. (ii) Historical change in the meaning of “aerogel” shows the recent situation with a flood of FD aerogels. (iii) The statistics show a division between traditional SCD-/APD-based studies and a recent fever with FD-based adsorbents, steam generation, sensors, electromagnetic wave shields, multifunctionality, etc., papers.
The data-driven analysis on polymer aerogels shows positive/negative aspects: (i) We can construct data infrastructure from the literature. The regression analysis decently supports conventional knowledge on aerogel chemistry. This approach would be beneficial for future prediction/exploring studies for high-performance aerogel design. (ii) There has been a certain amount of unreliable literature data. Actually, several researchers have already warned of the unreliable thermal conductivity issue from the ∼2010s in the aerogel community. Recent studies, including the current review, have visualized how serious this issue is. Researchers related to aerogels and other thermal insulators can no longer ignore this issue for reliable progress in the communities.
Finally, we suggest that the current “data-driven” review would be one type of next-generation review article. Reviews have long been written basically with accumulated knowledge by researchers in the field, while no one can escape from their biased ways of view. Such biases can be minimized by sticking to data. Besides, the data-driven approach needs an exhaustive survey, ∼18 000 papers checked one by one in the current case (it took 9 years since one of the authors started listing the papers), which is not easy in many fields. We expect progress in AI-based text mining technology; in the near future, such AI can probably generate a dataset from the web more accurately than human-handed works. However, as the current review has partly revealed, a data-driven approach has its limits on (i) the quality/bias of data and (ii) difficulty in unreliable data detection. Thus, accumulated conventional knowledge from researchers in the fields is continuously important for a successful data-driven approach.
After the explosion of AI-based technologies in the recent decade, many researchers would now agree with the benefits of a data-driven approach for saving time, effort, and resources in materials science with minimized human-derived biases. But we have to recognize that such an approach will one day be classified as unbalanced. The border between original articles and reviews could also be diminished. We hope our current review targeting aerogels, promising functional materials in the 21st century, would be the first and a good example of data-driven reviews, which could provide one case study for future discussion on how academic studies should be conducted.
Supporting Information
Details and additional figures for the analysis and literature lists are available in the Supporting Information. The dataset and ML codes (python scripts) can be found online at authors’ institutional repository (https://aist.repo.nii.ac.jp/records/2003338).
Acknowledgements
S.T. and T.O. appreciate the support from JSPS KAKENHI Grant Number JP22H01894/23K23162. S.M. appreciates the support from JSPS KAKENHI Grant Number JP24K17564. The authors appreciate Ms. Motomi Takehara for assisting literature data curation and organization.
Conflict of Interests
The authors declare no conflict of interest.
Biographies

Dr. Satoru Takeshita is a senior researcher at the National Institute of Advanced Industrial Science and Technology (AIST) of Japan. He received his PhD from Keio Univ. (2011), followed by 4 years postdoc/assistant. He joined AIST in 2015 and invented the first transparent chitosan aerogel. He was a guest scientist at the Swiss Federal Laboratories for Materials Science and Technology (EMPA) in 2019–2020. His research backbone lies in the chemistry of nanomaterials and currently focuses on the fundamental science and industrialization of biopolymer-based aerogels.

Dr. Shinya Mine is a researcher at the National Institute of Advanced Industrial Science and Technology (AIST). He received his PhD from Osaka Prefecture Univ. (2020), followed by 3 years as a postdoc at Hokkaido Univ. He joined AIST in 2023 and is working on the development of heterogeneous catalysts for effective CO2 reduction, making use of machine learning, high-throughput experiments, and DFT calculations. The backbone of his research is the mechanism elucidation of catalytic reactions based on DFT calculations. He is currently interested in applying data-driven materials development supported by machine learning to fields other than catalysts.

Dr. Takumi Ono is a senior researcher at the National Institute of Advanced Industrial Science and Technology (AIST). He received his PhD from Tohoku Univ. (2012), followed by 6 years as a postdoc. He joined AIST in 2018 and has worked on data-driven design of nanomaterial processes, including aerogels, nanocellular polymers, and machine-learning production of nanoparticles. His research backbone is the physicochemistry of solvents under high temperature and pressure. He currently focuses on data-driven materials development based on fluid property control.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.