Phenacetin[3]Arenes: Mannich-Type Macrocyclization, Unique Structure, Versatile Functionalization, and Strong Allosteric Binding
Yanling Shen
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXiaotong Liang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorTianning Ma
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dayang Zhou
Comprehensive Analysis Center, ISIR and Department of Applied Chemistry, Osaka University, Yamada-oka, Suita, 565-0871 Japan
Search for more papers by this authorWenjia Liu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorJingyu Ma
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Wanhua Wu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Zhipeng Yu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Cheng Yang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorYanling Shen
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorXiaotong Liang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorTianning Ma
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorProf. Dayang Zhou
Comprehensive Analysis Center, ISIR and Department of Applied Chemistry, Osaka University, Yamada-oka, Suita, 565-0871 Japan
Search for more papers by this authorWenjia Liu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorJingyu Ma
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorCorresponding Author
Prof. Wanhua Wu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Zhipeng Yu
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Cheng Yang
Key Laboratory of Green Chemistry and Technology of Ministry of Education, College of Chemistry and State Key Laboratory of Biotherapy, Sichuan University, Chengdu, 610064 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
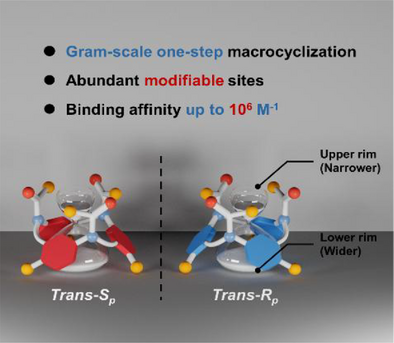
The one-step acid-catalyzed condensation of phenacetin and its analogues with paraformaldehyde provides a facile route to hourglass-shaped phenacetin[3]arenes with distinct functionalized rims in good yields. These novel macrocyclic arene show excellent binding properties and offer the potential for chirality sensing.
Abstract
This work introduces a novel NAm–CH₂–CAr macrocyclization pathway, diverging from the conventional CAr–CH₂–CAr linkages prevalent in macrocyclic arenes. This approach involves a one-pot condensation of - Phenacetin and its homologs with formaldehyde, yielding phenacetin[3]arenes (Ph[3]s) in yields up to 25.9%. Ph[3] exhibits an unsymmetrical hourglass-shaped architecture, featuring an upper rim adorned with amide groups and a lower rim comprising an alkoxylbenzene cavity. This unique structure facilitates reversible equilibrium between conformers via benzene ring flipping, which simultaneously reverses the orientation of amide groups, establishing equilibrium between C3 and F conformers. Increasing concentrations of organic ammonium guests lead to a transition from a predominantly 1:1 to 1:2 host–guest complexation. The estimated binding constants for the 1:1 complexes are in the order of 104–105 M−1, the overall binding constants for the 1:2 complexes are greater than 106 M−2. This stepwise complexation triggers a conformational shift from the C3 to F conformer, demonstrating intriguing allosteric behavior. Furthermore, interactions with chiral guests selectively influence the equilibrium of planar chiral conformers, generating chiroptical responses suitable for chirality sensing applications. The distinct functional groups on the two rims facilitate diverse chemical modifications, including reduction, deprotection, and condensation, providing synthetic flexibility for post-chemical modifications.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202504211-sup-0001-SuppMat.docx26.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. R. Wu, Y. W. Yang, Chem. Commun. 2019, 55, 1533–1543.
- 2Q. Shi, X. Wang, B. Liu, P. Qiao, J. Li, L. Wang, Chem. Commun. 2021, 57, 12379–12405.
- 3X. N. Han, Y. Han, C. F. Chen, Chem. Soc. Rev. 2023, 52, 3265–3298.
- 4C. B. Du, Y. J. Long, X. N. Han, Y. Han, C. F. Chen, Chem. Commun. 2024, 60, 13492–13506.
- 5A. Zinke, E. Ziegler, Ber. Dtsch. Chem. Ges. 1944, 77, 264–272.
10.1002/cber.19440770322 Google Scholar
- 6T. Ogoshi, S. Kanai, S. Fujinami, T. A. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 2008, 130, 5022–5023.
- 7M. Xue, Y. Yang, X. D. Chi, Z. B. Zhang, F. H. Huang, Acc. Chem. Res. 2012, 45, 1294–1308.
- 8S. T. Schneebeli, C. Cheng, K. J. Hartlieb, N. L. Strutt, A. A. Sarjeant, C. L. Stern, J. F. Stoddart, Chem.-Eur. J. 2013, 19, 3860–3868.
- 9X. N. Han, Y. Han, C. F. Chen, J. Am. Chem. Soc. 2020, 142, 8262–8269.
- 10X. N. Han, Y. Han, C. F. Chen, Nat. Commun. 2021, 12, 6378.
- 11W. Jiang, Y. Wang, Chin. J. Org. Chem. 2021.
- 12X. N. Han, Q. S. Zong, Y. Han, C. F. Chen, CCS Chem 2022, 4, 318–330.
- 13G. W. Zhang, P. F. Li, Z. Meng, H. X. Wang, Y. Han, C. F. Chen, Angew. Chem. Int. Ed. 2016, 55, 5304–5308; Angew. Chem. 2016, 128, 5390–5394.
- 14C. F. Chen, Y. Han, Acc. Chem. Res. 2018, 51, 2093–2106.
- 15J. Li, X. N. Han, H. Y. Zhou, Y. Han, C. F. Chen, J. Org. Chem. 2020, 85, 11465–11474.
- 16B. Li, B. Wang, X. Huang, L. Dai, L. Cui, J. Li, X. Jia, C. Li, Angew. Chem. Int. Ed. 2019, 131, 3885–3889; Angew. Chem. 2019, 131, 3925–3929.
- 17Y. Wang, K. Xu, B. Li, L. Cui, J. Li, X. Jia, H. Zhao, J. Fang, C. Li, Angew. Chem. Int. Ed. 2019, 58, 10281–10284; Angew. Chem. 2019, 131, 10387–10390.
- 18K. Xu, Z. Y. Zhang, C. Yu, B. Wang, M. Dong, X. Zeng, R. Gou, L. Cui, C. Li, Angew. Chem. Int. Ed. 2020, 132, 7214–7218; Angew. Chem. 2020, 132, 7281–7285.
- 19Z. Y. Zhang, C. Li, Acc. Chem. Res. 2022, 55, 916–929.
- 20T. Boinski, A. Cieszkowski, B. Rosa, A. Szumna, J. Org. Chem. 2015, 80, 3488–3495.
- 21B. Hua, L. Shao, J. Zhou, G. c. Yu, New J. Chem. 2016, 40, 4756–4760.
- 22J. Zhou, G. Yu, Q. Li, M. Wang, F. Huang, J. Am. Chem. Soc. 2020, 142, 2228–2232.
- 23J. R. Wu, Y. W. Yang, J. Am. Chem. Soc. 2019, 141, 12280–12287.
- 24J. R. Wu, Y. Wang, Y. W. Yang, Small 2020, 16, 2003490.
- 25P. Della Sala, R. Del Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. De Rosa, A. Spinella, A. Soriente, P. Neri, C. Gaeta, J. Am. Chem. Soc. 2020, 142, 1752–1756.
- 26R. Del Regno, P. Della Sala, D. Picariello, C. Talotta, A. Spinella, P. Neri, C. Gaeta, Org. Lett. 2021, 23, 8143–8146.
- 27P. Della Sala, R. Del Regno, L. Di Marino, C. Calabrese, C. Palo, C. Talotta, S. Geremia, N. Hickey, A. Capobianco, P. Neri, C. Gaeta, Chem. Sci. 2021, 12, 9952–9961.
- 28R. Del Regno, G. D. G. Santonoceta, P. Della Sala, M. De Rosa, A. Soriente, C. Talotta, A. Spinella, P. Neri, C. Sgarlata, C. Gaeta, Org. Lett. 2022, 24, 2711–2715.
- 29J. Li, H. Y. Zhou, Y. Han, C. F. Chen, Angew. Chem. Int. Ed. 2021, 60, 21927–21933; Angew. Chem. 2021, 133, 22098–22104.
- 30X. S. Du, D. W. Zhang, Y. Guo, J. Li, Y. Han, C. F. Chen, Angew. Chem. Int. Ed. 2021, 60, 13021–13028; Angew. Chem. 2021, 133, 13131–13138.
- 31J. Q. Wang, Y. Han, C. F. Chen, Chem. Commun. 2021, 57, 3987–3990.
- 32X. N. Han, P. F. Li, Y. Han, C. F. Chen, Angew. Chem. Int. Ed. 2022, 61, e202202527; Angew. Chem. 2022, 134, e202202527.
- 33Y. Zafrani, Y. Cohen, Org. Lett. 2017, 19, 3719–3722.
- 34P. Kumar, P. Venkatakrishnan, Org. Lett. 2018, 20, 1295–1299.
- 35J. Pfeuffer-Rooschüz, L. Schmid, A. Prescimone, K. Tiefenbacher, JACS Au 2021, 1, 1885–1891.
- 36W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof, A. C. Sue, Angew. Chem. Int. Ed. 2020, 59, 3994–3999; Angew. Chem. 2020, 132, 4023–4028.
- 37W. Yang, H. Wang, R. Chang, Z. Feng, Y. Zhu, A. C. Sue, Chem. Commun. 2023, 59, 2457–2460.
- 38Z. Xu, W. Yang, H. Liu, S. Jiang, A. C. H. Sue, JACS Au 2024, 4, 3475–3483.
- 39X. Y. Chen, H. Chen, J. F. Stoddart, Angew. Chem. Int. Ed. 2023, 62, e202211387; Angew. Chem. 2023, 135, e202211387.
- 40Y. Lu, S. M. Wang, S. S. He, Q. Huang, C. D. Zhao, S. Yu, W. Jiang, H. Yao, L. L. Wang, L. P. Yang, Chem. Sci. 2024, 15, 14791–14797.
- 41P. Spenst, F. Würthner, J. Photochem. Photobiol. C 2017, 31, 114–138.
- 42L. Mao, Y. Hu, Q. Tu, W. L. Jiang, X. L. Zhao, W. Wang, D. Yuan, J. Wen, X. Shi, Nat. Commun. 2020, 11, 5806.
- 43P. Jin, W. Liang, Y. Rong, W. Wu, M. Gou, Y. Tang, C. Yang, J. Mater. Chem. A 2023, 11, 11126–11132.
- 44W. Fang, J. Zhang, M. Guo, Y. Zhao, A. C. Sue, Angew. Chem. Int. Ed. 2024, 63, e202409120; Angew. Chem. 2024, 136, e202409120.
- 45P. Yang, Y. Jian, X. Zhou, G. Li, T. Deng, H. Shen, Z. Yang, Z. Tian, J. Org. Chem. 2016, 81, 2974–2980.
- 46C. Liu, Z. Li, H. Yu, N. Cui, X. Liao, H. Zhang, Z. Shu, P. Yang, Chin. Chem. Lett. 2021, 32, 1385–1389.
- 47F. Zhang, X. S. Du, D. W. Zhang, Y. F. Wang, H. Y. Lu, C. F. Chen, Angew. Chem. Int. Ed. 2021, 60, 15291–15295; Angew. Chem. 2021, 133, 15419–15423.
- 48H. Zhang, H. Li, S. Sun, L. Tan, H. Shen, B. Lin, P. Yang, Org. Lett. 2023, 25, 2078–2083.
- 49X. Yu, W. Wu, D. Zhou, D. Su, Z. Zhong, C. Yang, CCS Chem 2022, 4, 1806–1814.
- 50L. Mao, F. Li, L. Huang, X. Qu, K. Wang, Y. Zhang, D. Ma, Org. Lett. 2023, 25, 5597–5601.
- 51H. Y. Zhou, D. W. Zhang, M. Li, C. F. Chen, Angew. Chem. Int. Ed. 2022, 61, e202117872; Angew. Chem. 2022, 134, e202117872.
- 52H. Y. Zhou, D. W. Zhang, X. N. Han, Y. Han, C. F. Chen, Chem. Commun. 2022, 58, 12180–12183.
- 53N. Xue, H. Y. Zhou, Y. Han, M. Li, H. Y. Lu, C. F. Chen, Nat. Commun. 2024, 15, 1425.
- 54M. Schmidt, M. Hermann, F. Otteny, B. Esser, Org. Mater 2020, 02, 235–239.
- 55L. Mao, M. Zhou, T. Wu, D. Ma, G. Dai, X. Shi, Org. Lett. 2024, 26, 7244–7248.
- 56Y. Sun, L. Jiang, L. Liu, Y. Chen, W. Xu, J. Niu, Y. Qin, X. Xu, Y. Liu, Adv. Opt. Mater. 2023, 11.
- 57S. An, K. Gong, C. Yang, J. Su, Z. Zhang, Chemistry 2024, 30, e202400305.
- 58A. F. Strassberger, M. D. Zengaffinen, J. Puigcerver, N. Trapp, K. Tiefenbacher, Org. Lett. 2024, 26, 6720–6724.
- 59X. Liang, T. Zhao, Y. Shen, L. Fang, L. Chen, D. Zhou, W. Wu, C. Yang, Angew. Chem. Int. Ed. 2024, e202416975; Angew. Chem. 2025, 137, e202416975.
- 60Y. Mao, J. Ma, J. c. Ji, Y. Wang, W. Wu, C. Yang, Chin. Chem. Lett. 2024, 35, 109927.
- 61 A one-pot, two-step tandem reaction to obtain a fully reduced or fully hydrolyzed product (see the Supporting Information).
- 62CCDC 2424545 (for Ph[3]), 2424541/2424542 (for BzPh[3]), 2424546 (for BzPh[3]Me), 2424544 (for 3Bn-PAE[3]) and 2424543 (for PAE[3]) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 63C. Wolf, Dynamic Stereochemistry of Chiral Compounds: Principles and Applications, The Royal Society of Chemistry, London 2007.
- 64T. Ogoshi, K. Kitajima, T. Aoki, S. Fujinami, T. A. Yamagishi, Y. Nakamoto, J. Org. Chem. 2010, 75, 3268–3273.
- 65T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 66T. Lu, Q. Chen, J. Comput. Chem. 2022, 43, 539–555.
- 67T. Lu, J. Chem. Phys. 2024, 161.
- 68X. Liang, W. t. Liang, P. Jin, H. Wang, W. Wu, C. Yang, Chemosensors 2021, 9, 279.
- 69J. S. S. K. Formen, J. R. Howard, E. V. Anslyn, C. Wolf, Angew. Chem. Int. Ed. 2024, 63, e202400767; Angew. Chem. 2024, 136, e202400767.
- 70L. Alderighi, P. Gans, A. Ienco, D. Peters, A. Sabatini, A. Vacca, Coord. Chem. Rev. 1999, 184, 311–318.
- 71J. Ji, Y. Li, C. Xiao, G. Cheng, K. Luo, Q. Gong, D. Zhou, J. J. Chruma, W. Wu, C. Yang, Chem. Commun. 2020, 56, 161–164.