Vibronic Trimer Design Enhancing Intramolecular Triplet-Exciton Hopping to Accelerate Triplet-Triplet Annihilation for Photon Upconversion
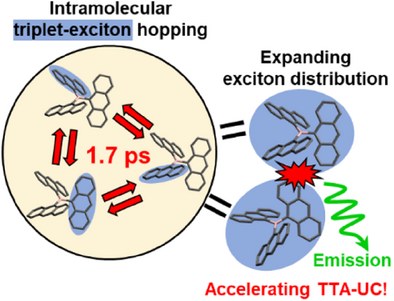
Graphical Abstract
Triplet-triplet annihilation-based photon up-conversion (TTA-UC) system using an anthracene-based UC emission by assembling multiple chromophores linked to a boron to enhance TTA rate constant. Structural fluctuations driven by vibronic motion induce picosecond intramolecular triplet exciton hopping, expanding the triplet exciton distribution and enhancing intermolecular TTA reactivity.
Abstract
Photon upconversion via triplet-triplet annihilation (TTA-UC) is a well-known process that converts low-energy light into higher-energy light. This process has attracted attention for its potential in various fields, including light-emitting devices, power generation and medical applications. It is desirable to develop TTA-UC materials of TTA emitters with large TTA rate constants (kTTA). However, molecular design to accelerate the bimolecular rate constant of kTTA has not been considered. We present a strategy to manipulate kTTA by assembling multiple chromophores linked to a boron in a rotationally symmetric manner, causing asymmetric motions by a localized triplet exciton. We have studied tri(9-anthryl)borane, which consists of three anthracenes linked via boron, as a TTA emitter. Time-resolved luminescence measurements confirmed that kTTA is improved compared to the conventional TTA-UC system using DPA, an anthracene-based monomer. Time-resolved electron paramagnetic resonance measurements showed that the improvement in kTTA is due to fast intramolecular triplet exciton hopping coupled with vibrational motions in the trimer molecule, which extends the reactivity at the collision distance between the excitons through the pseudo-rotational motions. This molecular design that enhances TTA reactivity is expected to contribute to the future development of TTA-UC materials for sensing fluid environment.
Introduction
As a means to effectively utilize limited energy resources, light energy conversion technology is an indispensable element in modern society. Among them, photon upconversion via triplet-triplet annihilation (TTA-UC) is a chemical reaction process that converts two photons into one higher-energy photon.[1, 2] This process operates even when incoherent low-intensity light is incident, making it possible to effectively utilize light of various wavelengths contained in sunlight. Therefore, TTA-UC attracts attention as a promising means to improve the light energy conversion efficiency in organic solar cells[3, 4] and photocatalysis.[5, 6] Furthermore, because TTA-UC can realize the light-energy conversion using weak excitation lights compatible with the human body, it is expected to be useful in various medical fields.[7-12] A typical TTA-UC system often consists of a combination of a sensitizer and an TTA emitter molecule and goes through a complex process.[13] TTA is a bimolecular reaction whereby two triplet-excitons collide. Hence the longer the lifetime (τT) of the triplet-exciton and the larger the TTA reaction rate (kTTA), the higher the quantum yields (ΦTTA) of TTA becomes. A conventional approach to prolong τT is to control the molecular structure to suppress nonradiative deactivation and excimer formation processes.[14-17]
For example, 9,10-diphenylanthracene (DPA), which is used as an TTA emitter in the TTA-UC systems, has very long τT, over 1 ms,[18] and achieves high ΦTTA. On the other hand, molecular design focusing to improve the kTTA is also important but has not been paid significant attention. When we only consider intramolecular TTA,[19-21] we do not need to discuss diffusion rate because the collisions between chromophore molecules are not needed for TTA. However, molecular diffusion is often required for several practical TTA-UC applications in fluid environments.
To increase kTTA in the solution-based TTA-UC, it is necessary to either increase the diffusion rate of the emitter molecule or to enhance the effective TTA reaction zone between the triplet-excitons, that is, the spatial extent of unpaired orbitals in the triplet-exciton. Solution TTA-UC materials using low-viscosity solvents such as toluene and tetrahydrofuran have been reported in numerous studies.[15, 20, 22-24] When the molecular diffusion rate is larger than kTTA, it is important to enhance the reactivity between the triplet excitons for improving kTTA.[15, 25, 26] When kTTA is the diffusion-controlled rate constant, it might be better to use a lower viscosity solvent, but will be difficult to find a solvent that can dramatically improve kTTA. Enhancing the TTA reaction distance would be plausible when triplet-excitons possesses highly delocalized unpaired orbitals. However, this simple expansion of the orbitals generally lowers the triplet energy level, causing difficulty in obtaining the radiative first excited singlet state (S1emi) from the two of the first excited triplet states (T1) as in the case of triplet-pair by pentacene, which is well known as a singlet-fission material.
Instead, if we can prepare a molecule containing multiple chromophores with a small core structure that has high rotational symmetry, we can expect to realize a situation where the triplet-exciton moves quickly between chromophores in the entire molecule by a pseudo-rotation caused by the dynamic Jahn-Teller effect in highly symmetric molecules such as fullerenes.[27-31] Thus, it is expected that the unpaired orbitals in the triplet-exciton can be equivalent to be delocalized during the time scale of the bimolecular triplet-triplet encounter by the collision without lowering the triplet energy, thereby improving kTTA.[32-34]
For this, one needs to control the molecular motion by selecting the central atom bridging the chromophores. For example, Crystal Violet (CV) is a trimer with aniline chromophores linked by a central carbon that satisfies the octet rule. CV shows strong charge-transfer (CT) characteristics and takes on a stable planar structure in the triplet state.[35] On the other hand, in the case of bulky trimeric chromophores linked with a boron atom, the octet rule is not satisfied. This instability by the low bond-order from the B atom is expected to lead to the molecular activation motions such as pseudo-rotation of triplet excitons within the trimer. To prove the concept of the above-mentioned strategy to improve kTTA, we have employed a TTA-UC material of tri(9-anthryl)borane (TAB),[36, 37] which is a trimer-type fluorescent molecule that is expected to exhibit triplet-exciton hopping within the molecule with the addition of the photosensitizer, platinum 2,3,7,8,12,13,17,18-octaethylporphyrin (PtOEP).[17, 20, 21] The TTA-UC emission from TAB is investigated by time-resolved fluorescence measurement to determine kTTA in the PtOEP/TAB system. The estimated kTTA value is compared with that in PtOEP/DPA system, which is well known for its high overall UC quantum yield (ΦUC ≈ 24%),[20, 38] to verify the effect of exciton dynamics by the trimerization on kTTA. Furthermore, the electronic structure of the triplet-exciton generated in TAB is investigated by using time-resolved electron paramagnetic resonance (TREPR), and it is clarified from a molecular point of view how the molecular structure and mobility contribute to the improvement of kTTA.
Results and Discussion
To characterize excited state energies, Figure 1a shows the absorption and emission spectra of PtOEP, TAB, and DPA along with their molecular structures. TAB has a unique structure with three anthracenes linked via boron in a three-dimensionally orthogonal arrangement. TAB was synthesized by a previously reported method.[37] Due to the interaction between the three anthracene moieties, the absorption and fluorescence wavelengths of TAB are red-shifted compared to DPA, which is one of the anthracene monomer derivatives. This is ascribed to a CT type transition between HOMO and LUMO as shown by the DFT calculation in Figure S1.
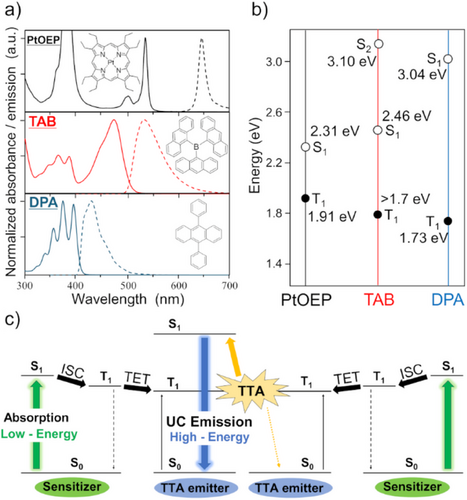
Figure 1b shows the energy levels of the first excited singlet state (S1) and the first excited triplet state (T1) of each molecule. The S1 and T1 energies of PtOEP shown in Figure 1b are based on reported values.[39] PtOEP absorbs green light and exhibits a high ISC yield (ΦISC ≈ 1) due to the heavy atom effect,[40] making its energy relationship with anthracene derivatives suitable for the TTA-UC system. The S1 energies of TAB and DPA were estimated from the intersection of the absorption and fluorescence spectra in Figure 1a, which is consistent with a TD-SCF calculation (Figure S1). The S2 energy of TAB was estimated from the edge of the short-wavelength absorption band (400 nm). The T1 energies of TAB (>1.7 eV) and DPA (1.73 eV) were estimated based upon the TTA-UC observation via TTE from TAB to DPA (Figure S2). For DPA, the calculated T1 energy[20] coincided with experimentally determined value.[41] From Figure 1b, the TTA is expected to proceed from the T1T1 pair (reactant energy: > 3.4 eV) to the S1S0 state (product energy: 2.46 eV) in TAB.
Figure 1c shows the Jablonski diagram of the TTA-UC system composed of two photosensitizers and two emitters. The T1 energy of PtOEP is higher than those of DPA and TAB, indicating that the TET reactions from PtOEP to DPA or TAB occur exothermically. Additionally, because the S1 energies of DPA and TAB are lower than twice of their respective T1 energies, the energy requirements of TTA-UC are satisfied when both DPA and TAB are used as the TTA emitters. Compared to the TTA-UC material composed of PtOEP and DPA, the PtOEP/TAB system exhibits a smaller anti-Stokes shift. This is because the orbital distributions of HOMO and LUMO in the TAB molecule extend over two or three anthracene moieties via the boron atom (Figure S1) to cause a CT character, resulting in a lowered S1 energy level.
To determine TTA rate constants, the photon upconversion processes of the two different TTA emitters were examined using time-resolved photoluminescence measurements at room temperature. Solutions consisting of 60 µM PtOEP and 1.2 mM TAB or DPA were prepared in toluene and were degassed by freeze-pump-thaw cycles. Figure 2a and b show the time profiles of the TTA-UC emission intensities for the PtOEP/TAB and PtOEP/DPA systems, respectively, detected after excitation by laser pulse at 532 nm at various intensities. In both systems, as the excitation light intensity increases, the emission decays faster, indicating that the emission occurs through a bimolecular reaction process as illustrated in the Figure 1c.
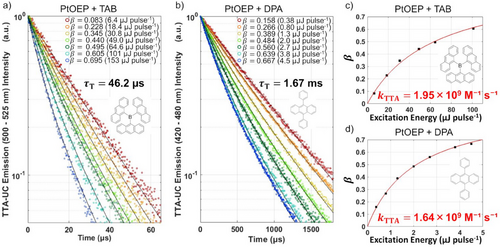
[3Emi*]0 produced by the TET (t = 0) was estimated from the incident light intensity and the photophysical parameters of PtOEP. Solid black curves in Figure 2a and b show the results of least squares fittings to determine the τT and β values those of which are shown in these figures.
The β value ranges from 0 to 1, and β/2 equals to the ΦTTA. Solid red curves in Figure 2c and d are the results of least squares fittings using Equations (1) and (2), and kTTA values of TAB and DPA shown in these figures are determined, respectively.
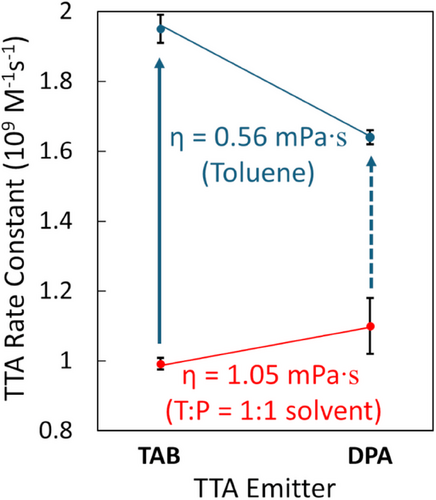
We also determined the kTTA values in a viscous solvent of a mixture of toluene(T): paraffin(P) = 1:1 (v/v) (Figure S4, Table S2). kTTA of TAB was slightly lower or unchanged from that of DPA, as shown by the red plots in Figure 3. This reduction of kTTA for TAB in the viscous solvent would be caused by prolonging the pseudo-rotational time of the triplet-exciton. We note that this reversal is not explained by the Franck-Condon factor for the TTA (vide infra).
To elucidate the accelerated and deaccelerated kTTAs in TAB at the molecular level from spin-state dynamics of the triplet-exciton, we performed TREPR measurements of the PtOEP/TAB and PtOEP/DPA systems. PtOEP and either TAB or DPA were dissolved in solvents with different viscosities prepared by mixing low viscosity toluene and high viscosity liquid paraffin in various mixing ratios.[50, 51] The sample solutions were degassed and sealed in 5 mm quartz EPR tubes. As in the time-resolved emission measurements, a Nd:YAG laser at 532 nm was used for light excitation. Importantly, the triplet state of PtOEP is not detected in the X-band (∼ 9.5 GHz) EPR used in this study because of its large zero-field splitting (ZFS) interaction.
There are advantages to performing the EPR measurements under varying viscosity conditions. In high viscosity solutions, the rotation of the molecules is slow enough so that the anisotropy by the ZFS is observed with the random orientations. As a result, the motional narrowing effect on the EPR line-shape is suppressed, and EPR spectra similar to those obtained in frozen solutions are observed, allowing for determination of ZFS parameters: D and E.[52, 53] As the viscosity of the solvent decreases, the degrees of freedom related to molecular motion, including rotation and vibrations, are increased. This leads to a high rotation frequency of the molecule comparable to the frequency of the ZFS interaction. Thermally activated intramolecular exciton hopping driven by the molecular vibrations may also induce pseudo-rotation as a temperature dependent process. These exciton dynamics can be observed as the motional narrowing effects on the EPR spectral line-shapes.[54]
Figure 4 shows the TREPR spectra of PtOEP/TAB system and PtOEP/DPA system in solvents of varying viscosity at room temperature. Spectral line shapes reflect the spin populations of the triplet sublevels (|T+〉, |T0〉, |T−〉) of emitters, by the TET from PtOEP. Thermal equilibrium electron spin population according to the Boltzmann distribution is assumed here to interpret the microwave-absorptive EPR spectra, although the absorptive spin polarization could possibly be created by the spin-selective reactions in the triplet-pairs during the TTA process, as reported previously.[55] The slower rise and longer decay time of the EPR signal with increasing viscosity is attributed to the slower rate of TET and TTA, respectively, due to slower molecular diffusion.
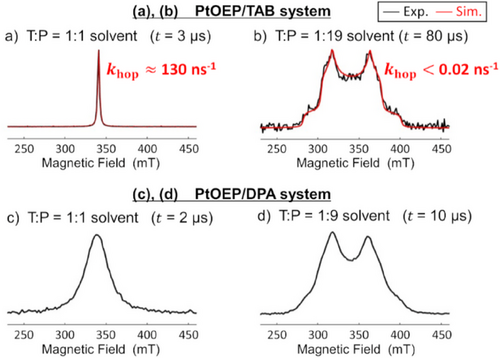
In PtOEP/TAB system (Figures 4a, S5–S7), the EPR linewidth was narrowed in the mixture of toluene(T): paraffin(P) = 1:1 (v/v) solvent due to a rapid change in the orientation of triplet-exciton relative to the external magnetic field. In contrast, in the T:P = 1:19 solvent, the narrowing effect is suppressed, and broad EPR spectra were observed due to the anisotropy of the spin-spin dipole interaction (Figure 4b). In the T:P = 1:9 mixture of the PtOEP/DPA system (Figure 4d), the motional narrowing is suppressed, as well as in the PtOEP/TAB system with T:P = 1:19. In these viscous solutions, the total splitting widths of the spectra (c.a.120 mT) by the fine structures are comparable for both TAB and DPA. The spin density is distributed to approximately one, locally excited (LE) anthracene in TAB, as in DPA (Figures 5, S8). The similarity of the spin distribution means that the ZFS parameter in DPA is similar to those in TAB, which is consistent with the similar widths by the dipolar splitting in Figure 4b and d, although a motional narrowing effect on the spectrum would slightly influence the line shape.
Comparing the EPR spectra between Figure 4a and c in the T:P = 1:1 solvent, the linewidth of the EPR spectrum of the PtOEP/TAB system is about 3 mT (Figure 4a), while the linewidth of the EPR spectrum of the PtOEP/DPA system is about 30 mT (Figure 4c). This small linewidth for the TAB system should originate from the narrowing effect due to more rapid rotational motions of triplet-exciton of TAB than that of DPA in the presence of the magnetic field, because the ZFS couplings were similar in the viscous media. Typically, molecules with small molecular radii are expected to rotate quickly, leading to more prominent motional narrowing effects. The quick rotation of triplet-excitons on the TAB is thus due to the pseudo-rotation rather than molecular rotation. Therefore, we hypothesize that the intramolecular triplet-exciton hopping occurs within TAB[17, 32-34] and thus contributes to the enhanced kTTA. On the other hand, we surmise that the 30 mT width in Figure 4c is caused by the molecular rotation diffusion in 3DPA* because no exciton hopping is expected at the dilute DPA concentration in the fluid.
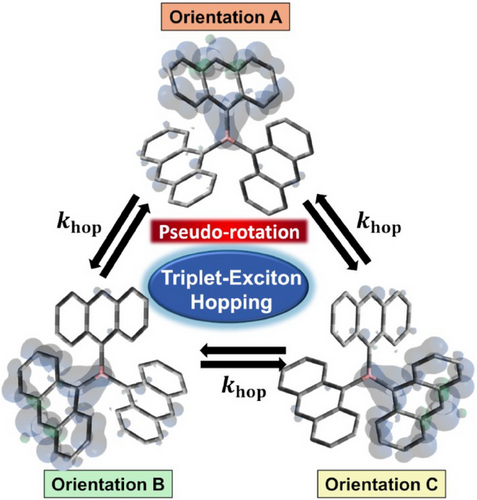
The spin density distribution of the triplet-exciton of TAB is shown in Figure 5, which is predicted by geometry optimizations as detailed in Figure S11. Since the three anthracene moieties of TAB are arranged in a 3D orthogonal relationship, the hopping causes three orientations of the triplet-exciton to rotate approximately by 90° about two of the axes (x, y, z) within a coordinate frame in the molecule (Figure 5). Such a conformation jump in the orientation of the triplet-exciton leads to motional narrowing of the EPR spectra as the pseudo-rotation, which is effectively equivalent to molecular rotation.[27-29, 32, 54-56]
Given that the conformation distortion by the local exciton residence is minor (Figure S9b) and thus symmetry of the TAB molecule is approximately preserved due to its trimeric structure, it is expected that there are three equivalent sites where the triplet-exciton is distributed, and we assumed that the triplet-exciton hops between these sites (orientations A, B and C) at the hopping rate constant “khop”.[55, 56] Details are described in the Supporting Informational on the EPR spectrum analysis using the coupled stochastic-Liouville equations (SLE) considering the A⇄B, B⇄C, and C⇄A jumps by khop in the rotating frame with Figures S10–S15 and Tables S3–S6. Validities of the fitting parameters are examined as detailed in Table S6 and Figures S16–S18.
The results of the line shape analyses by the above SLE are shown in Figure 4 where the khop values for TAB are described. khop = 130 ns−1 in the bulky 3TAB* molecule suggests that the rotation of triplet excitons is due to intramolecular exciton hopping in a much shorter timescale than the molecular rotation dynamics observed in the small molecule of 3DPA*. As the molecular rotation of DPA is suppressed by increasing viscosity (Figure 4d), the khop value of TAB is also small in the T:P = 1:19 solvent (Figure 4b), indicating that the intramolecular exciton hopping is hindered. The reason for this hinderance is detailed in Figure S9b.
The free energy change (ΔGTTA) for the TTA is evaluated to be − 0.9 eV using ΔGTTA = E(S1) − 2E(T1) in Figure 1b. The reorganization energy of λ (= 2λT) for T1 + T1 → S0 + S1 is estimated to be > 0.80 eV by which conformations of two of the triplet excitons are rearranged to those of the two singlets. From ΔGTTA + λ ≈ 0 eV, a large Franck-Condon factor is conclusive, as detailed in the Supporting Information. Thus, in the present exergonic TTA systems, the rate constant of kTTA is limited both by the molecular diffusion and by spin statistics, especially in the PtOEP/TAB system.
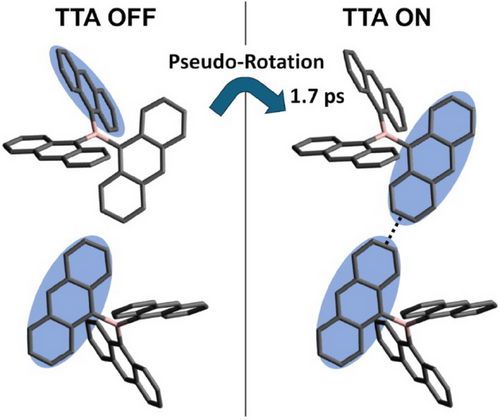
This means that the intramolecular exciton hopping avoids the mismatches at the collision distance, resulting in the TTA reaction rate being close to the diffusion-controlled rate. In contrast, the excitons are not able to change their orientation within the collision time of 2 ps in 3DPA*.
Contrary to the 2 ps hopping time of 3TAB* in toluene, tcollision ≪ τhop = 8 ps is obtained in the viscous solvent (η = 1.05 mPa) in Table S7. This supports the hopping-mediated TTA in toluene (η = 0.56 mPa) with tcollision = τhop, because kTTA(3TAB*) < kTTA(3DPA*) (red plots of Figure 3) denotes that the inhibited hopping results in the mismatch in the exchange interaction between the 3TAB* molecules to reduce kTTA significantly. The larger increase in kTTA(3TAB*) as shown by the solid arrow in Figure 3 than the increase represented by the dashed arrow is thus rationalized by the enhanced kTTA by the quick vibronic hopping in 3TAB*.
From this, we conclude that the picosecond intramolecular hopping impacts kTTA in the low viscous solution by expanding effective triplet spin distribution in the time scale of the molecular collision. These ultrafast hopping dynamics can be applicable for several solid-state materials and encapsulated emitter systems to enhance the TTA-UC processes. The weakness of TAB as a TTA emitter is its short triplet lifetime (46.2 µs), which will reduce the chance of the TTA given that the bimolecular kTTA rate constant is not highly influenced due to the diffusion-controlled limit in fluid. Our future task is to enhance kTTA further by designing bulky TTA emitters that lengthen tcollision, which competes with the vibronic hopping, and to develop TTA emitters with extended triplet lifetime while maintaining the improved kTTA.
Conclusion
In this study, we focused on accelerating the TTA rate constant, which has not been extensively studied before, by synthesizing a trimeric UC emission material, TAB. This material combines three anthracene molecules, which are expected to exhibit intramolecular triplet exciton hopping. We developed a TTA-UC material by combining TAB with one of the photosensitizers, PtOEP, and experimentally evaluated the TTA rate constant and the mobility of triplet excitons during the TTA-UC process using time-resolved luminescence measurements and time-resolved EPR measurements. We confirmed that the structural fluctuations driven by vibronic interactions in the TAB molecule facilitate the quick intramolecular migration of triplet excitons generated by the energy transfer reaction from PtOEP among the anthracene units on TAB, effectively increasing the TTA reactivity. The present picosecond vibronic motion is realized by introducing the asymmetric motions by a localized triplet exciton via the boron atom in TAB. The challenge is to develop new molecules that achieve both extended triplet lifetimes and TTA reaction distances by modifying molecular designs such as introducing substituents. This new strategy of utilizing trimeric molecules that induce long-distance intermolecular TTA is promising to accelerate the development of TTA-UC materials with high light-to-energy conversion efficiency.
Supporting Information
The authors have cited additional references within the Supporting Information.[60, 61]
Acknowledgements
This work was partially supported by JSPS KAKENHI Grant-in-Aid for Transformative Research Areas, “Dynamic Exciton (Area No. 20A201)” (JP20H05832) and Grant Nos. JP25H00903, JP23H00309, JP22H00344, JP23H03945E, JP23K17901, JP22K14648 and the JST-CREST Program (JPMJCR23I6). Y.K. and C.K. are grateful for support from Invitational Fellowships for Research in Japan (No. 1201456) by J.S.P.S. Y.K. and E.S. thank to Prof. Seigo Yamauchi (Tohoku University, deceased) on preliminary discussions of the exciton properties in TAB.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.