Utilizing the Mn(II) Dissolution-Induced Vacancy for Optimum Mg2+ Storage of Spinel Mn3O4
Graphical Abstract
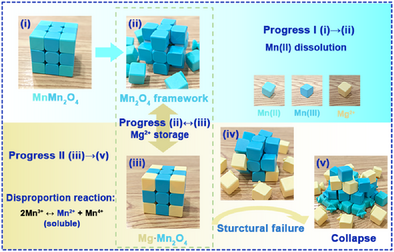
There are two significant factors necessary for the cathode materials of aqueous Mg-ion energy storage devices as follows: one is the storage mechanism of Mg2+ that can achieve enhanced Mg2+ diffusion kinetics and bring higher power density; the other is the high structural stability to achieve long lifespan. The tetrahedral vacancy reversible insertion/extraction mechanism by Mn(II) dissolution and optimize the integrity of the spinel framework is a powerful strategy to achieve Mg2+ energy storage system with high rate performance and long lifespan.
Abstract
Manganese-based oxide can theoretically exert the multivalent advantages of an aqueous magnesium-ion cathode due to its redox activity and abundant crystal structure. However, sluggish diffusion kinetics of Mg2+ and Mn dissolution limit the rate performance and structure stability. Herein, we successfully utilize the notorious dissolution of Mn(II) tetrahedral site contributed vacancies for packaging optimum Mg2+ storage of a popular spinel Mn3O4 electrode. Such mechanism reverses the sluggish diffusion kinetics. Moreover, merited by the common ion effect and drug dissolution, a suitable preaddition of Mn2+ to electrolyte inhibit Mn(III) dissolution and optimize the integrity of the spinel framework. Impressively, the cathode achieves a reversible capacity of 310 mAh g−1 and a stable cycle performance of 2000 cycles with 94.9% retention. Our research shows that reversible insertion/extraction at vacancies and effective stabilization of spinel framework is a powerful strategy to achieve Mg2+ ion energy storage system with high rate performance and long lifespan.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in Supporting Information of this article.