Comparative Study of Solvatomorphs of Stryker's Reagent Using MicroED and Quantum Mechanics
Corresponding Author
Dr. Kunal K. Jha
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Jacob O. Rothbaum
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorVignesh C. Bhethanabotla
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorCharles B. Musgrave III
Division of Applied Physics and Material Science, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Christopher G. Jones
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Sergey I. Morozov
Department of Physics of Nanoscale Systems, South Ural State University, Chelyabinsk, 454080 Russia
Search for more papers by this authorCorresponding Author
Prof. William A. Goddard III
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Hosea M. Nelson
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Dr. Kunal K. Jha
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorDr. Jacob O. Rothbaum
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorVignesh C. Bhethanabotla
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorCharles B. Musgrave III
Division of Applied Physics and Material Science, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Christopher G. Jones
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
Search for more papers by this authorDr. Sergey I. Morozov
Department of Physics of Nanoscale Systems, South Ural State University, Chelyabinsk, 454080 Russia
Search for more papers by this authorCorresponding Author
Prof. William A. Goddard III
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Hosea M. Nelson
Division of Chemistry & Chemical Engineering, California Institute of Technology, Pasadena, CA, 91125 USA
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
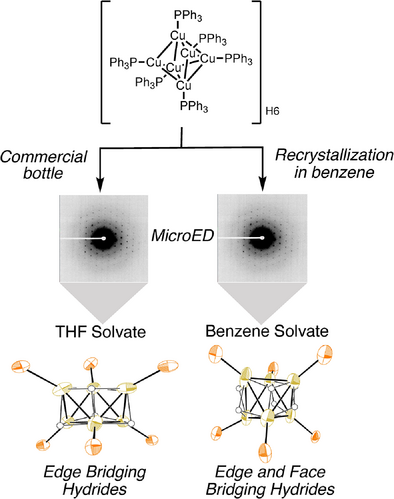
A new solvate form of Stryker's reagent was crystallized from benzene which has regular octahedra with equivalent Cu–Cu interatomic distances. Quantum crystallography approach HAR led to reliable position of hydrides in both the forms. The reported THF solvate form has edge bridging hydrides while the newly discovered form may have both edge and face bridging hydrides supported by structural and topological analysis.
Abstract
The atomic position of hydrogen atoms in metal hydrides has been a long-standing structural question in inorganic chemistry given that hydride delivery is integral to diverse chemical reactions. Microcrystal electron diffraction (microED), with it's increased sensitivity toward hydrogen atoms relative to X-ray diffraction, offers a potential path to addressing this challenge. Herein, the first microED study of Stryker's reagent is reported, resulting in the structure of a new benzene solvate. Improved accuracy for hydrogen atom positions was obtained via a quantum crystallography (QCr) approach, Hirshfeld atom refinement (HAR). Structural and topological analysis supports edge bridging hydrides in the microED structure of a THF solvate form, consistent with previous diffraction studies. Interestingly, analysis of a new benzene solvate, discovered in this study, is consistent with mixed edge- and face-bridging hydrides.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202502524-supp-0001-SuppMat.docx22.7 MB | Supporting Information |
| anie202502524-supp-0002-SuppMat.cif8.3 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. A. Bezman, M. R. Churchill, J. A. Osborn, J. Wormald, J. Am. Chem. Soc. 1971, 93, 2063–2065.
- 2M. R. Churchill, S. A. Bezman, J. A. Osborn, J. Wormald, Inorg. Chem. 1972, 11, 1818–1825.
- 3W. S. Mahoney, D. M. Brestensky, J. M. Stryker, J. Am. Chem. Soc. 1988, 110, 291–293.
- 4D. M. Brestensky, D. E. Huseland, C. McGettigan, J. M. Stryker, Tetrahedron Lett. 1988, 29, 3749–3752.
- 5D. M. Brestensky, J. M. Stryker, Tetrahedron Lett. 1989, 30, 5677–5680.
- 6T. M. Koenig, J. F. Daeuble, D. M. Brestensky, J. M. Stryker, Tetrahedron Lett. 1990, 31, 3237–3240.
- 7J. Chen, J. F. Daeuble, J. M. Stryker, Tetrahedron 2000, 56, 2789–2798.
- 8P. Chiu, S. K. Leung, Chem Comm 2004, 20, 2308.
- 9A. de Fátima, Synlett 2005, 11, 1805–1806.
- 10A. I. Meyers, T. R. Elworthy, J. Org. Chem. 1992, 57, 4732–4740.
- 11P. Chiu, C. P. Szeto, Z. Geng, K. F. Cheng, Tetrahedron Lett. 2001, 42, 4091–4093.
- 12S. Diethelm, E. M. Carreira, J. Am. Chem. Soc. 2015, 137, 6084–6096.
- 13C. F. Heinrich, C. Peter, L. Miesch, P. Geoffroy, M. Miesch, Synthesis 2016, 48, 1607–1615.
- 14T. H. Lemmen, K. Folting, J. C. Huffman, K. G. Caulton, J. Am. Chem. Soc. 1985, 107, 7774–7775.
- 15D. M. Ho, R. Bau, Inorg. Chim. Acta 1984, 84, 213–220.
- 16R. C. Stevens, M. R. McLean, R. Bau, T. F. Koetzle, J. Am. Chem. Soc. 1989, 111, 3472–3473.
- 17E. L. Bennett, P. J. Murphy, S. Imberti, S. F. Parker, Inorg. Chem. 2014, 53, 2963–2967.
- 18M. Schmidtmann, P. Coster, P. F. Henry, V. P. Ting, M. T. Weller, C. C. Wilson, CrystEngComm 2014, 16, 1232–1236.
- 19P. Müller, R. Herbst-Irmer, A. L. Speck, T. R. Schneider, M. R. Sawaya, in Crystal Structure Refinement: A Crystallographer's Guide to SHELXL, IuCr Texts on Crystallography, Chap. 3, Oxford University Press, 2006.
10.1093/acprof:oso/9780198570769.001.0001 Google Scholar
- 20C. G. Jones, M. W. Martynowycz, J. Hattne, T. J. Fulton, B. M. Stoltz, J. A. Rodriguez, H. M. Nelson, T. Gonen, ACS Cent. Sci. 2018, 4, 1587–1592.
- 21M. T. B. Clabbers, M. W. Martynowycz, J. Hattne, T. Gonen, J. Struc. Biol. 2022, 6, 100078.
- 22M. T. B. Clabbers, T. Gruene, E. van Genderen, J. P. Abrahams, Acta Cryst. A. 2019, 75, 82–93.
- 23C. G. Jones, M. Asay, L. J. Kim, J. F. Kleinsasser, A. Saha, T. J. Fulton, K. R. Berkley, D. Cascio, A. G. Malyutin, M. P. Conley, B. M. Stoltz, V. Lavallo, J. A. Rodríguez, H. M. Nelson, ACS Cent. Sci. 2019, 5, 1507–1513.
- 24T. Gruene, J. J. Holstein, G. H. Clever, B. Keppler, Nat. Rev. Chem. 2021, 5, 660–668.
- 25D. Watkin, Acta Cryst. A 1994, 50, 411–437.
- 26B. Dittrich, IUCrJ 2021, 8, 305–318.
- 27L. Palatinus, P. Brázda, P. Boullay, O. Perez, M. Klementová, S. Petit, V. Eigner, M. Zaarour, S. Mintova, Science 2017, 355, 166–169.
- 28W. Sun, P. Chen, M. Zhang, J. Ma, J. Sun, Angew. Chem. Int. Ed. 2023, 62, e20230598.
- 29F. Kleemiss, O. V. Dolomanov, M. Bodensteiner, N. Peyerimhoff, L. Midgley, L. J. Bourhis, A. Genoni, L. A. Malaspina, D. Jayatilaka, J. L. Spencer, F. White, B. Grundkötter-Stock, S. Steinhauer, D. Lentz, H. Puschmann, S. Grabowsky, Chem. Sci. 2021, 12, 1675–1692.
- 30R. F. Stewart, E. R. Davidson, W. T. Simpson, J. Chem. Phys. 1965, 42, 3175–3187.
- 31R. F. Stewart, J. Bentley, B. Goodman, J. Chem. Phys. 1975, 63, 3786–3793.
- 32F. L. Hirshfeld, Theor. Chim. Acta 1977, 44, 129–138.
- 33D. Jayatilaka, B. Dittrich, Acta Cryst. A 2008, 64, 383–393.
- 34P. M. Dominiak, A. Volkov, X. Li, M. Messerschmidt, P. Coppens, J. Chem. Theory Comput. 2007, 3, 232–247.
- 35M. Woińska, S. Grabowsky, P. M. Dominiak, K. Woźniak, D. Jayatilaka, Sci. Adv. 2016, 2, e1600192.
- 36K. K. Jha, B. Gruza, P. Kumar, M. L. Chodkiewicz, P. M. Dominiak, Acta Cryst. B. 2020, 76, 296–306.
- 37K. K. Jha, B. Gruza, M. L. Chodkiewicz, C. Jelsch, P. M. Dominiak, J. Appl. Cryst. 2021, 54, 1234–1243.
- 38L. Baharudin, A. C. K. Yip, V. B. Golovko, M. I. J. Polson, M. J. Watson, Chem. Eng. J. 2019, 377, 120278.
- 39D. C. Sass, V. C. G. Heleno, S. Cavalcante, J. Da Silva Barbosa, A. C. F. Soares, M. G. Constantino, J. Org. Chem. 2012, 77, 9374–9378.
- 40S. K. Wolff, D. J. Grimwood, J. J. McKinnon, D. Jayatilaka, M. A. Spackman, Crystal Explorer, University of Western Australlia, Perth, Australia, 2007.
- 41A. L. Speck, Acta Cryst. C. 2015, C71, 9–18.
- 42K. K. Jha, F. Kleemiss, M. L. Chodkiewicz, P. M. Dominiak, J. Appl. Cryst. 2023, 56, 116–127.
- 43Deposition numbers 2338306, 2336736, 2336737, 2336738, 2336739, 2336740 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service. The data containing topological analysis table are available at https://doi.org/10.5281/zenodo.11111181.
10.5281/zenodo.11111181 Google Scholar
- 44R. F. W. Bader, Acc. Chem. Res. 1985, 18, 9–15.
- 45P. K. Mehrotra, R. Hoffmann, Inorg. Chem. 1978, 17, 2187–2189.
- 46M. A. Carvajal, S. Alvarez, J. J. Novoa, Chem. - Eur. J. 2004, 10, 2117–2132.
- 47S. Dinda, A. G. Samuelson, Chem. - Eur. J. 2012, 18, 3032–3042.
- 48T. H. Kim, Y. W. Shin, J. W. Jung, J. S. Kim, J. Kim, Angew. Chem. Int. Ed. 2008, 47, 685–688.
- 49V. W. Yam, V. K. Au, S. Y. Leung, Chem. Rev. 2015, 115, 7589–7728.
- 50W. Kabsch, Acta Cryst. D 2010, 66, 125–132.
- 51W. Kabsch, Acta Cryst. D 2010, 66, 133–144.
- 52J. Hattne, F. E. Reyes, B. L. Nannenga, D. Shi, M. J. de la Cruz, A. G. W. Leslie, T. Gonen, Acta Cryst. A. 2015, 71, 353–360.
- 53G. M. Sheldrick, Acta Cryst. A. 2015, 71, 3–8.
- 54O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Cryst. 2009, 42, 339–341.
- 55L. M. Peng, Micron 1999, 30, 625–648.
- 56I. A. Guzei, J. Appl. Cryst. 2014, 47, 806–809.
- 57P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka, M. A. Spackman, J. Appl. Cryst. 2021, 54, 1006–1011.
- 58M. A. Spackman, J. J. Mckinnon, CrystEngComm 2002, 4, 378–392.
- 59F. Neese, F. Wennmohs, U. Becker, C. Riplinger, J. Chem. Phys. 2020, 152, 224108.
- 60C. R. Groom, I. J. Bruno, M. P. Lightfoot, S. C. Ward, Acta Cryst. B. 2016, 72, 171–179.
- 61T. Lu, F. Chen, J. Comput. Chem. 2012, 33, 580–592.
- 62Y. Zhao, D. G. Truhlar, J. Chem. Phys. 2006, 125, 194101.
- 63V. Barone, M. Cossi, J. Phys. Chem. A 1998, 102, 1995–2001.
- 64G. Kresse, J. Hafner, Phys. Rev. B 1993, 47, 558–561.
- 65G. Kresse, J. Furthmüller, Comput. Mat. Sci. 1996, 6, 15–50.
- 66G. Kresse, J. Furthmüller, Phys. Rev. B 1996, 54, 11169–11186.
- 67G. Kresse, J. Hafner, J. Phys.: Condens. Matter 1994, 6, 8245–8257.
- 68G. Kresse, D. Joubert, Phys. Rev. B 1999, 59, 1758–1775.
- 69J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 70S. Nosé, J. Chem. Phys. 1984, 81, 511–519.