Rhodium-Catalyzed Atroposelective Synthesis of Axially Chiral 1-Aryl Isoquinolines via De Novo Isoquinoline Formation
Bo-Bo Gou
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorWen-Jie Shen
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Yuan-Jun Gao
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorQing Gu
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Shu-Li You
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorBo-Bo Gou
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorWen-Jie Shen
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Yuan-Jun Gao
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorQing Gu
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
Search for more papers by this authorCorresponding Author
Shu-Li You
New Cornerstone Science Laboratory, State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai, 200032 China
E-mail: [email protected]; [email protected]
Search for more papers by this authorDedicated to Professor Xue-Long Hou on the occasion of his 70th birthday
Homepage: http://shuliyou.sioc.ac.cn/
Graphical Abstract
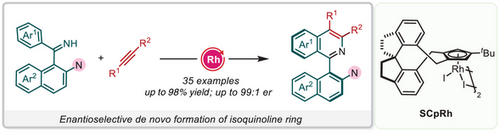
A highly efficient synthesis of axially chiral 1-aryl isoquinolines through rhodium-catalyzed C─H activation/annulation of aromatic imines with alkynes is reported. An enantioselective de novo isoquinoline ring formation is involved in this process. Detailed experimental and theoretical studies revealed the turnover-limiting step of the reaction and the origin of enantioselectivity.
Abstract
Axially chiral heterobiaryl moieties serve as core skeletons for bioactive molecules, chiral ligands, and organocatalysts. Enantioselective de novo formation of the heteroaromatic ring is one of the most straightforward approaches to access enantioenriched heterobiaryls. Herein, an enantioselective de novo construction of isoquinolines by rhodium-catalyzed C─H activation/annulation of aromatic imines with alkynes is disclosed. This approach is operationally simple, allowing for rapid access to a variety of axially chiral 1-aryl isoquinolines in excellent yields and enantioselectivity (up to 98% yield and 99:1 er). The synthetic application of the current method was demonstrated by functional group transformations and suitability for millimolar-scale reactions. Detailed experimental and theoretical studies revealed the turnover-limiting step and provided insight into the origin of the enantioselectivity for this reaction.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article
Supporting Information
| Filename | Description |
|---|---|
| anie202502131-sup-0001-SuppMat.pdf20.7 MB | Supporting Information |
| anie202502131-sup-0002-SuppMat.cif1.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. C. Kozlowski, B. J. Morgan, E. C. Linton, Chem. Soc. Rev. 2009, 38, 3193–3207.
- 2G. Bringmann, T. Gulder, T. A. M. Gulder, M. Breuning, Chem. Rev. 2011, 111, 563–639.
- 3J. E. Smyth, N. M. Butler, P. A. Keller, Nat. Prod. Rep. 2015, 32, 1562–1583.
- 4S. R. LaPlante, P. J. Edwards, L. D. Fader, A. Jakalian, O. Hucke, ChemMedChem 2011, 6, 505–513.
- 5S. R. LaPlante, L. D. Fader, K. R. Fandrick, D. R. Fandrick, O. Hucke, R. Kemper, S. P. F. Miller, P. J. Edwards, J. Med. Chem. 2011, 54, 7005–7022.
- 6M. P. Carroll, P. J. Guiry, Chem. Soc. Rev. 2014, 43, 819–833.
- 7E. Kumarasamy, R. Raghunathan, M. P. Sibi, J. Sivaguru, Chem. Rev. 2015, 115, 11239–11300.
- 8B. V. Rokade, P. J. Guiry, ACS Catal. 2018, 8, 624–643.
- 9S. B. Cortright, J. N. Johnston, Angew. Chem. Int. Ed. 2002, 41, 345–348.
10.1002/1521-3773(20020118)41:2<345::AID-ANIE345>3.0.CO;2-U CAS PubMed Web of Science® Google Scholar
- 10A. V. Malkov, L. Dufková, L. Farrugia, P. Kočovský, Angew. Chem. Int. Ed. 2003, 42, 3674–3677.
- 11S. B. Cortright, J. C. Huffman, R. A. Yoder, J. N. Coalter, J. N. Johnston, Organometallics 2004, 23, 2238–2250.
- 12A. V. Malkov, P. Ramírez-López, L. Biedermannová, L. Rulíšek, L. Dufková, M. Kotora, F. Zhu, P. Kočovský, J. Am. Chem. Soc. 2008, 130, 5341–5348.
- 13S. Tanaka, T. Seki, M. Kitamura, Angew. Chem. Int. Ed. 2009, 48, 8948–8951.
- 14J. Francos, F. Grande-Carmona, H. Faustino, J. Iglesias-Sigüenza, E. Díez, I. Alonso, R. Fernández, J. M. Lassaletta, F. López, J. L. Mascareñas, J. Am. Chem. Soc. 2012, 134, 14322–14325.
- 15E. Fernández, P. J. Guiry, K. P. T. Connole, J. M. Brown, J. Org. Chem. 2014, 79, 5391–5400.
- 16J. Wencel-Delord, A. Panossian, F. R. Leroux, F. Colobert, Chem. Soc. Rev. 2015, 44, 3418–3430.
- 17B. Zilate, A. Castrogiovanni, C. Sparr, ACS Catal. 2018, 8, 2981–2988.
- 18J. A. Carmona, C. Rodríguez-Franco, R. Fernández, V. Hornillos, J. M. Lassaletta, Chem. Soc. Rev. 2021, 50, 2968–2983.
- 19J. K. Cheng, S.-H. Xiang, S. Li, L. Ye, B. Tan, Chem. Rev. 2021, 121, 4805–4902.
- 20R. Miyaji, K. Asano, S. Matsubara, J. Am. Chem. Soc. 2015, 137, 6766–6769.
- 21A. Romero-Arenas, V. Hornillos, J. Iglesias-Sigüenza, R. Fernández, J. López-Serrano, A. Ros, J. M. Lassaletta, J. Am. Chem. Soc. 2020, 142, 2628–2639.
- 22B. Yang, J. Gao, X. Tan, Y. Ge, C. He, Angew. Chem. Int. Ed. 2023, 62, e202307812.
- 23M. Xiong, Z. Yan, S.-C. Chen, J. Tang, F. Yang, D. Xing, ACS Catal. 2024, 14, 7243–7255.
- 24J. Zheng, W.-J. Cui, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5242–5245.
- 25Q. Wang, W.-W. Zhang, H. Song, J. Wang, C. Zheng, Q. Gu, S.-L. You, J. Am. Chem. Soc. 2020, 142, 15678–15685.
- 26W.-W. Zhang, C.-X. Liu, P. Yang, S.-Z. Zhang, Q. Gu, S.-L. You, Org. Lett. 2022, 24, 564–569.
- 27W.-W. Zhang, Q. Wang, S.-Z. Zhang, C. Zheng, S.-L. You, Angew. Chem. Int. Ed. 2023, 62, e202214460.
- 28D.-S. Zheng, W.-W. Zhang, Q. Gu, S.-L. You, ACS Catal. 2023, 13, 5127–5134.
- 29D.-S. Zheng, P.-P. Xie, F. Zhao, C. Zheng, Q. Gu, S.-L. You, ACS Catal. 2024, 14, 6009–6015.
- 30V. Bhat, S. Wang, B. M. Stoltz, S. C. Virgil, J. Am. Chem. Soc. 2013, 135, 16829–16832.
- 31A. Ros, B. Estepa, P. Ramírez-López, E. Álvarez, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2013, 135, 15730–15733.
- 32V. Hornillos, A. Ros, P. Ramírez-López, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, Chem. Commun. 2016, 52, 14121–14124.
- 33P. Ramírez-López, A. Ros, B. Estepa, R. Fernández, B. Fiser, E. Gómez-Bengoa, J. M. Lassaletta, ACS Catal. 2016, 6, 3955–3964.
- 34P. Ramírez-López, A. Ros, A. Romero-Arenas, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2016, 138, 12053–12056.
- 35S. Staniland, R. W. Adams, J. J. W. McDouall, I. Maffucci, A. Contini, D. M. Grainger, N. J. Turner, J. Clayden, Angew. Chem. Int. Ed. 2016, 55, 10755–10759.
- 36J. A. Carmona, V. Hornillos, P. Ramírez-López, A. Ros, J. Iglesias-Sigüenza, E. Gómez-Bengoa, R. Fernández, J. M. Lassaletta, J. Am. Chem. Soc. 2018, 140, 11067–11075.
- 37V. Hornillos, J. A. Carmona, A. Ros, J. Iglesias-Sigüenza, J. López-Serrano, R. Fernández, J. M. Lassaletta, Angew. Chem. Int. Ed. 2018, 57, 3777–3781.
- 38P.-Y. Jiang, K.-F. Fan, S. Li, S.-H. Xiang, B. Tan, Nat. Commun. 2021, 12, 2384.
- 39X. Jiang, W. Xiong, S. Deng, F.-D. Lu, Y. Jia, Q. Yang, L.-Y. Xue, X. Qi, J. A. Tunge, L.-Q. Lu, W.-J. Xiao, Nat. Catal. 2022, 5, 788–797.
- 40H. Dong, C. Wang, J. Am. Chem. Soc. 2023, 145, 26747–26755.
- 41T. Sun, Z. Zhang, Y. Su, H. Cao, Y. Zhou, G. Luo, Z.-C. Cao, J. Am. Chem. Soc. 2023, 145, 15721–15728.
- 42W. Xiong, X. Jiang, W.-C. Wang, Y. Cheng, L.-Q. Lu, K. Gao, W.-J. Xiao, J. Am. Chem. Soc. 2023, 145, 7983–7991.
- 43P.-Y. Jiang, S. Wu, G.-J. Wang, S.-H. Xiang, B. Tan, Angew. Chem. Int. Ed. 2023, 62, e202309272.
- 44Á. Mosquera, M. A. Pena, J. Pérez Sestelo, L. A. Sarandeses, Eur. J. Org. Chem. 2013, 2013, 2555–2562.
- 45D. Shen, Y. Xu, S.-L. Shi, J. Am. Chem. Soc. 2019, 141, 14938–14945.
- 46A. Gutnov, B. Heller, C. Fischer, H.-J. Drexler, A. Spannenberg, B. Sundermann, C. Sundermann, Angew. Chem. Int. Ed. 2004, 43, 3795–3797.
- 47L. Zhang, J. Shen, S. Wu, G. Zhong, Y.-B. Wang, B. Tan, Angew. Chem. Int. Ed. 2020, 59, 23077–23082.
- 48G. Yang, S. Sun, Z. Li, Y. Liu, J. Wang, Commun. Chem. 2021, 4, 144.
- 49D. Moser, K. Jana, C. Sparr, Angew. Chem. Int. Ed. 2023, 62, e202309053.
- 50G. Wang, X. Tan, B.-X. Yan, Z.-W. Zhang, G. Luo, Z.-S. Ye, J. Am. Chem. Soc. 2024, 146, 27809–27818.
- 51Q.-Q. Yang, C. Chen, D. Yao, W. Liu, B. Liu, J. Zhou, D. Pan, C. Peng, G. Zhan, B. Han, Angew. Chem. Int. Ed. 2024, 63, e202312663.
- 52C. G. Newton, S.-G. Wang, C. C. Oliveira, N. Cramer, Chem. Rev. 2017, 117, 8908–8976.
- 53J. Loup, U. Dhawa, F. Pesciaioli, J. Wencel-Delord, L. Ackermann, Angew. Chem. Int. Ed. 2019, 58, 12803–12818.
- 54Ł. Woźniak, N. Cramer, Trends Chem. 2019, 1, 471–484.
- 55T. K. Achar, S. Maiti, S. Jana, D. Maiti, ACS Catal. 2020, 10, 13748–13793.
- 56N. Y. S. Lam, K. Wu, J.-Q. Yu, Angew. Chem. Int. Ed. 2021, 60, 15767–15790.
- 57Y. Sun, N. Cramer, Angew. Chem. Int. Ed. 2017, 56, 364–367.
- 58G. Shan, J. Flegel, H. Li, C. Merten, S. Ziegler, A. P. Antonchick, H. Waldmann, Angew. Chem. Int. Ed. 2018, 57, 14250–14254.
- 59F. Wang, Z. Qi, Y. Zhao, S. Zhai, G. Zheng, R. Mi, Z. Huang, X. Zhu, X. He, X. Li, Angew. Chem. Int. Ed. 2020, 59, 13288–13294.
- 60Q. Wang, Y.-H. Nie, C.-X. Liu, W.-W. Zhang, Z.-J. Wu, Q. Gu, C. Zheng, S.-L. You, ACS Catal. 2022, 12, 3083–3093.
- 61H. Liang, W. Guo, J. Li, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2022, 61, e202204926.
- 62G. Zhou, T. Zhou, A.-L. Jiang, P.-F. Qian, J.-Y. Li, B.-Y. Jiang, Z.-J. Chen, B.-F. Shi, Angew. Chem. Int. Ed. 2024, 63, e202319871.
- 63W. Guo, J. Jiang, J. Wang, Angew. Chem. Int. Ed. 2024, 63, e202400279.
- 64F. Wang, J. Jing, Y. Zhao, X. Zhu, X.-P. Zhang, L. Zhao, P. Hu, W.-Q. Deng, X. Li, Angew. Chem. Int. Ed. 2021, 60, 16628–16633.
- 65B. Ye, N. Cramer, Acc. Chem. Res. 2015, 48, 1308–1318.
- 66C. G. Newton, D. Kossler, N. Cramer, J. Am. Chem. Soc. 2016, 138, 3935–3941.
- 67S. Shaaban, C. Davies, H. Waldmann, Eur. J. Org. Chem. 2020, 2020, 6512–6524.
- 68T. Yoshino, S. Satake, S. Matsunaga, Chem. - Eur. J. 2020, 26, 7346–7357.
- 69C. Pan, S.-Y. Yin, Q. Gu, S.-L. You, Org. Biomol. Chem. 2021, 19, 7264–7275.
- 70T. Yoshino, S. Matsunaga, ACS Catal. 2021, 11, 6455–6466.
- 71C. Davies, S. Shaaban, H. Waldmann, Trends Chem. 2022, 4, 318–330.
- 72C.-X. Liu, S.-Y. Yin, F. Zhao, H. Yang, Z. Feng, Q. Gu, S.-L. You, Chem. Rev. 2023, 123, 10079–10134.
- 73T. Fukutani, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Commun. 2009, 5141–5143.
- 74C. Pan, S.-Y. Yin, S.-B. Wang, Q. Gu, S.-L. You, Angew. Chem. Int. Ed. 2021, 60, 15510–15516.
- 75S.-Y. Yin, C. Pan, W.-W. Zhang, C.-X. Liu, F. Zhao, Q. Gu, S.-L. You, Org. Lett. 2022, 24, 3620–3625.
- 76 Deposition number 2415769 (for 3aa) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 77C. Liu, H. Sun, C. Qin, T. Yang, W. Zhang, Y. Zhou, Y. Li, Z. R. Jia, C. Chu, Synlett 2022, 33, 993–997.