Imine Reductase–Catalyzed Remote Stereocontrol for Enantiodivergent Synthesis of Cyclohexylidene-Based Axially Chiral Amines
Keting Li
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Both authors contributed equally to this work.
Search for more papers by this authorZhen Liu
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Bin Wang
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorDr. Ling Huang
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorLuyao Yu
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorZitian Zhou
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorCorresponding Author
Prof. Liang Lin
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Pengfei Fang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Haigen Fu
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorKeting Li
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Both authors contributed equally to this work.
Search for more papers by this authorZhen Liu
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Both authors contributed equally to this work.
Search for more papers by this authorDr. Bin Wang
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorDr. Ling Huang
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorLuyao Yu
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorZitian Zhou
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
Search for more papers by this authorCorresponding Author
Prof. Liang Lin
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Pengfei Fang
State Key Laboratory of Chemical Biology, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, University of Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorCorresponding Author
Prof. Haigen Fu
NHC Key Laboratory of Biotechnology for Microbial Drugs, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050 China
E-mail: [email protected]; [email protected]; [email protected]
Search for more papers by this authorGraphical Abstract
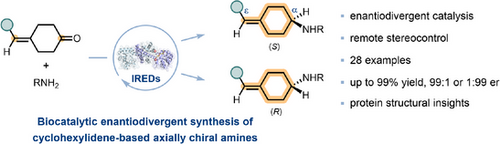
A biocatalytic route for the enantiodivergent synthesis of cyclohexylidene-based axially chiral amines was developed. This method features imine reductase catalyzed remote stereocontrol in establishing the unusual axial chirality arising from the restricted double bond, providing both enantiomers of chiral products in high yield with high enantioselectivity.
Abstract
Cyclohexylidene-based amines exhibit unique axial chirality arising from the restricted double bond and have shown great potential in medicinal chemistry. However, their asymmetric synthesis remains challenging due to the long distance between the chirally relevant groups. Herein, we report a highly efficient and asymmetric synthesis of cyclohexylidene-based axially chiral amines from 4-substituted cyclohexanones and primary amines catalyzed by imine reductases (IREDs). Enantiodivergent IREDs were identified to provide convenient access to both enantiomers of chiral products with high yields and enantioselectivity (up to 99% yield, 99:1 or 1:99 enantiomeric ratio). A gram-scale synthesis of cyclohexylidene-based amines was also achieved. Moreover, protein X-ray crystallography and molecular modeling studies were conducted to provide structural insight into the remote stereocontrol of IREDs in generating cyclohexylidene-based axial chirality.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in Supporting Information of this article.
Supporting Information
| Filename | Description |
|---|---|
| anie202500572-sup-0001-SuppMat.pdf11.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. A. McGrath, M. Brichacek, J. T. Njardarson, J. Chem. Educ. 2010, 87, 1348–1349.
- 2D. E. Smith, I. Marquez, M. E. Lokensgard, A. L. Rheingold, D. A. Hecht, J. L. Gustafson, Angew. Chem. Int. Ed. 2015, 54, 754–11759.
- 3M. Basilaia, M. H. Chen, J. Secka, J. L. Gustafson, Acc. Chem. Res. 2022, 55, 2904–2919.
- 4K. V. Rao, W. P. Cullen, Antibiot. Annu. 1959, 7, 950–953.
- 5K. V. Rao, K. Biemann, R. B. Woodward, J. Am. Chem. Soc. 1963, 85, 2532–2533.
- 6N. N. Nasir, M. Sekar, S. Ravi, L. S. Wong, S. P. Sisinthy, S. H. Gan, V. Subramaniyan, K. Chidambaram, N. N. I. M. Rani, M. Y. Begum, M. Ramar, S. Z. Safi, S. Selvaraj, S. K. C. Maruthu, S. Fuloria, N. K. Fuloria, P. T. Lum, S. Djearamane, Drug Des. Devel. Ther. 2023, 17, 1065–1078.
- 7G. Bringmann, Y. Reichert, V. V Kane, Tetrahedron 2004, 60, 3539–3574.
- 8T. J. Donohoe, C. R. Jones, L. C. A. Barbosa, J. Am. Chem. Soc. 2011, 133, 418–16421.
- 9L. F. Valdez Pérez, S. P. J. T. Bachollet, N. V Orlov, K. P. M. Kopf, J. P. A. Harrity, Angew. Chem. Int. Ed. 2023, 62, e202213692.
- 10N. Tajuddeen, D. Feineis, H. Ihmels, G. Bringmann, Acc. Chem. Res. 2022, 55, 2370–2383.
- 11Y. J. Wu, Z. K. Wang, Z. S. Jia, J. H. Chen, F. R. Huang, B. B. Zhan, Q. J. Yao, B. F. Shi, Angew. Chem. Int. Ed. 2023, 62, e202310004.
- 12B. B. Zhan, L. Wang, J. Luo, X. F. Lin, B. F. Shi, Angew. Chem. Int. Ed. 2020, 59, 3568–3572.
- 13K. Mori, T. Itakura, T. Akiyama, Angew. Chem. Int. Ed. 2016, 55, 642–11646.
- 14J. Zhang, J. Wang, Angew. Chem. Int. Ed. 2018, 57, 465–469.
- 15J. A. Carmona, C. Rodríguez-Franco, J. López-Serrano, A. Ros, J. Iglesias-Sigüenza, R. Fernández, J. M. Lassaletta, V. Hornillos, ACS Catal. 2021, 11, 4117–4124.
- 16C. Rodríguez-Franco, E. Roldán-Molina, A. Aguirre-Medina, R. Fernández, V. Hornillos, J. M. Lassaletta, Angew. Chem. Int. Ed. 2024, 63, e202409524.
- 17D. Bhuniya, R. K. Kharul, A. Hajare, N. Shaikh, S. Bhosale, S. Balwe, F. Begum, S. De, S. Athavankar, D. Joshi, V. Madgula, K. Joshi, A. A. Raje, A. V. Meru, A. Magdum, K. A. Mookhtiar, R. Barbhaiya, Bioorg. Med. Chem. Lett. 2019, 29, 238–243.
- 18Z. L. Wang, Y. H. Xu, Synthesis 2023, 56, 1259–1272.
- 19F. Hu, Y. Xia, Eur. J. Org. Chem. 2023, 26, e202300151.
- 20J. C. Fiaud, J. Y. Legros, Tetrahedron Lett. 1988, 29, 2959–2962.
- 21J. Y. Legros, J. C. Fiaud, Tetrahedron 1994, 50, 465–474.
- 22S. Li, J. L. Xu, Y. H. Xu, Org. Lett. 2022, 24, 6054–6059.
- 23C. Ma, Y. Sun, S. Liu, Z. M. Li, J. Yang, H. Guo, J. Zhang, Chem Catal. 2022, 2, 3196–3206.
- 24B. R. Shao, W.F. Jiang, C. Zheng, L. Shi, Chem Catal. 2023, 3, 100–697.
- 25J. Z. Essman, E. N. Jacobsen, J. Am. Chem. Soc. 2024, 146, 7165–7172.
- 26B.-R. Shao, W.-F. Jiang, C. Wang, L. Shi, Angew. Chem. Int. Ed. 2025, 64, e202421287.
- 27S. Arai, S. Hamaguchi, T. Shioiri, Tetrahedron Lett. 1998, 39, 2997–3000.
- 28L. Gramigna, S. Ducea, G. Filippini, M. Fochie, M. C. Franchini, L. Bernardi,Synlett 2011, 18, 2745–2749.
- 29M. Ying, K. Wang, W. Yan, M. Pu, L. Lin, Chem. Eur. J. 2024, 30, e202401243.
- 30S. Crotti, N. Di Iorio, C. Artusi, A. Mazzanti, P. Righi, G. Bencivenni, Org. Lett. 2019, 21, 3013–3017.
- 31S. K. Nimmagadda, S. C. Mallojjala, L. Woztas, S. E. Wheeler, J. C. Antilla, Angew. Chem. Int. Ed. 2017, 56, 2454–2458.
- 32S. Zhu, J. H. Mao, J. K. Cheng, S. H. Xiang, B. Tan, Chem 2022, 8, 2529–2541.
- 33R. Agudo, G. D. Roiban, M. T. Reetz, J. Am. Chem. Soc. 2013, 135, 1665–1668.
- 34A. J. Metrano, S. J. Miller, Acc. Chem. Res. 2019, 52, 199–215.
- 35T. S. Mei, H. H. Patel, M. S. Sigman, Nature 2014, 508, 340–344.
- 36N. U. D. Reshi, V. B. Saptal, M. Beller, J. K. Bera, ACS Catal. 2021, 11, 809–13837.
- 37I. P. Beletskaya, C. Nájera, M. Yus, Chem. Rev. 2018, 118, 5080–5200.
- 38R. Buller, S. Lutz, R. J. Kazlauskas, R. Snajdrova, J. C. Moore, U. T. Bornscheuer, Science 2024, 382, eadh8615.
- 39M. D. Patil, G. Grogan, A. Bommarius, H. Yun, ACS Catal. 2018, 8, 985–11015.
- 40T. C. Wang, B. K. Mai, Z. Zhang, Z. Bo, J. Li, P. Liu, Y. Yang, Nature 2024, 629, 98–104.
- 41M. Alfaro Blasco, H. Gröger, Bioorg. Med. Chem. 2014, 22, 5539–5546.
- 42M. Li, W. Harrison, Z. Zhang, Y. Yuan, H. Zhao, Nat. Chem. 2024, 16, 277–284.
- 43H. O'Dowd, J. L. Manske, S. A. Freedman, J. E. Cochran, Org. Lett. 2022, 24, 3431–3434.
- 44G. A. Aleku, ACS Catal. 2024, 14, 308–14329.
- 45J. Mangas-Sanchez, S. P. France, S. L. Montgomery, G. A. Aleku, H. Man, M. Sharma, J. I. Ramsden, G. Grogan, N. J. Turner, Curr. Opin. Chem. Biol. 2017, 37, 19–25.
- 46B. Yuan, D. Yang, G. Qu, N. J. Turner, Z. Sun, Chem. Soc. Rev. 2024, 53, 227–262.
- 47Z. Wang, G.-S. Gao, Y.-D. Gao, L.-C. Yang, Org. Process Res. Dev. 2024, 28, 3035–3054.
- 48K. Mitsukura, M. Suzuki, K. Tada, T. Yoshida, T. Nagasawa, Org. Biomol. Chem. 2010, 8, 4533–4535.
- 49M. Schober, C. MacDermaid, A. A. Ollis, S. Chang, D. Khan, J. Hosford, J. Latham, L. A. F. Ihnken, M. J. B. Brown, D. Fuerst, M. J. Sanganee, G.-D. Roiban, Nat. Catal. 2019, 2, 909–915.
- 50A. K. Gilio, T. W. Thorpe, N. Turner, G. Grogan, Chem. Sci. 2022, 13, 4697–4713.
- 51X. Hao, Z. Tian, Z. Yao, T. Zang, S. Song, L. Lin, T. Qiao, L. Huang, H. Fu, Angew. Chem. Int. Ed. 2024, 63, e202410112.
- 52P. Zhang, B. Yuan, J. Li, C. Li, J. Guo, B. Zhang, G. Qu, H. Su, N. J. Turner, Z. Sun, Angew. Chem. Int. Ed. 2024, 64, e202416569.
- 53B. Chen, R. Li, J. Feng, B. Zhao, J. Zhang, J. Yu, Y. Xu, Z. Xing, Y. Zhao, B. Wang, X. Huang, J. Am. Chem. Soc. 2024, 146, 278–14286.
- 54X. Hao, B. Wang, Z. Tian, Z. Yao, T. Qiao, L. Huang, H. Fu, Org. Chem. Front. 2025, 12, 2658-2669.
- 55J. Steflik, A. Gilio, M. Burns, G. Grogan, R. Kumar, R. Lewis, C. Martinez, ACS Catal. 2023, 13, 10065–10075.
- 56X. Li, Y. Hu, J. D. Bailey, B. H. Lipshutz, Org. Lett. 2024, 26, 2778–2783.
- 57M. Cortes-clerget, N. Akporji, J. Zhou, F. Gao, P. Guo, M. Parmentier, F. Gallou, J. Berthon, B. H. Lipshutz, Nat. Commun. 2019, 10, 2169.
- 58 Deposition numbers 2403393 (R-3), 2403398 (S-3), 2403471 (R-26) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallo-graphic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 59X.-X. Zhu, W.-Q. Zheng, Z.-W. Xia, X.-R. Chen, T. Jin, X.-W. Ding, F.-F. Chen, Q. Chen, J.-H. Xu, X.-D. Kong, G.-W. Zheng, Nat. Commun. 2024, 15, 10330.
- 60M. Rodríguez-Mata, A. Frank, E. Wells, F. Leipold, N. J. Turner, S. Hart, J. P. Turkenburg, G. Grogan, ChemBioChem 2013, 14, 1372–1379.
- 61T. Huber, L. Schneider, A. Präg, S. Gerhardt, O. Einsle, M. Müller, ChemCatChem 2014, 6, 2248–2252.
- 62G. A. Aleku, H. Man, S. P. France, F. Leipold, S. Hussain, L. Toca-Gonzalez, R. Marchington, S. Hart, J. P. Turkenburg, G. Grogan, N. J. Turner, ACS Catal. 2016, 6, 3880–3889.
- 63G. A. Aleku, S. P. France, H. Man, J. Mangas-Sanchez, S. L. Montgomery, M. Sharma, F. Leipold, S. Hussain, G. Grogan, N. J. Turner, Nat. Chem. 2017, 9, 961–969.
- 64A. T. McNutt, P. Francoeur, R. Aggarwal, T. Masuda, R. Meli, M. Ragoza, J. Sunseri, D. R. Koes, J. Cheminform. 2021, 13, 43.
- 65S. Velikogne, V. Resch, C. Dertnig, J. H. Schrittwieser, W. Kroutil, ChemCatChem 2018, 10, 3236–3246.
- 66K. Wu, J. Huang, L. Shao, ChemCatChem 2022, 14, e202200921.
- 67J. S. Kang, N. Kim, T. Kim, M. Seo, B. Kim, Macromol. Rapid Commun. 2022, 43, 2100649.
- 68R. Eelkema, B. L. Feringa, Org. Biomol. Chem. 2006, 4, 3729–3745.
- 69H. K. Bisoyi, Q. Li, Chem. Rev. 2016, 116, 089–15166.