Functionalized Cyclic Poly(α-Hydroxy Acids) via Controlled Ring-Opening Polymerization of O-Carboxyanhydrides
Ziyu Huo
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorXiaoyu Xie
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorNadim Mahmud
Department of Chemistry, Virginia Polytechnic Institute and State University, 1040 Drillfield Drive, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorJoshua C. Worch
Department of Chemistry, Virginia Polytechnic Institute and State University, 1040 Drillfield Drive, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorCorresponding Author
Rong Tong
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
E-mail: [email protected]
Search for more papers by this authorZiyu Huo
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorXiaoyu Xie
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorNadim Mahmud
Department of Chemistry, Virginia Polytechnic Institute and State University, 1040 Drillfield Drive, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorJoshua C. Worch
Department of Chemistry, Virginia Polytechnic Institute and State University, 1040 Drillfield Drive, Blacksburg, Virginia, 24061 USA
Search for more papers by this authorCorresponding Author
Rong Tong
Department of Chemical Engineering, Virginia Polytechnic Institute and State University, 635 Prices Fork Road, Blacksburg, Virginia, 24061 USA
E-mail: [email protected]
Search for more papers by this authorGraphical Abstract
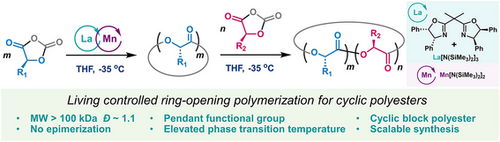
A novel catalytic system consisting of a lanthanum complex with a sterically bulky ligand and a manganese silylamide has been developed to prepare high-molecular-weight (>100 kDa) stereoregular cyclic polyesters and block copolyesters with narrow molecular weight distributions (Ɖ < 1.1) and unique physical properties.
Abstract
Linear poly(α-hydroxy acids) are important degradable polymers, and they can be efficiently prepared by ring-opening polymerization of O-carboxyanhydrides with pendant functional groups. However, attempts to prepare cyclic poly(α-hydroxy acids) have been plagued by side reactions, including epimerization and uncontrolled intramolecular chain transfers or termination, that prevent the synthesis of high-molecular-weight stereoregular cyclic polyesters. Herein, we report a scalable method for the synthesis of high-molecular-weight (>100 kDa) stereoregular functionalized cyclic poly(α-hydroxy acids) by means of controlled polymerization of O-carboxyanhydrides using a catalytic system consisting of a lanthanum complex with a sterically bulky ligand and a manganese silylamide. Additionally, using this system, we could readily prepare cyclic block poly(α-hydroxy acids) by means of sequential addition of O-carboxyanhydrides. The obtained cyclic polyesters and their cyclic block copolyesters exhibit distinctive physicochemical properties—including elevated phase transition temperature, improved toughness, and reduced viscosity—compared to their linear counterparts.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
| Filename | Description |
|---|---|
| anie202423973-sup-0001-SuppMat.pdf6.4 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Y. Tezuka, H. Oike, Prog. Polym. Sci. 2002, 27, 1069–1122.
- 2T. Yamamoto, Y. Tezuka, Polym. Chem. 2011, 2, 1930–1941.
- 3J. A. Semlyen, Cyclic polymers, 2nd ed., Kluwer Academic Publishers, Dordrecht, the Netherlands 2000.
- 4K. Endo, in New Frontiers in Polymer Synthesis (Ed.: S. Kobayashi), Springer, Berlin Heidelberg 2008, pp. 121–183.
10.1007/12_2008_157 Google Scholar
- 5F. M. Haque, S. M. Grayson, Nat. Chem. 2020, 12, 433–444.
- 6M. Kapnistos, M. Lang, D. Vlassopoulos, W. Pyckhout-Hintzen, D. Richter, D. Cho, T. Chang, M. Rubinstein, Nat. Mater. 2008, 7, 997–1002.
- 7J. Roovers, P. M. Toporowski, Macromolecules 1983, 16, 843–849.
- 8B. A. Laurent, S. M. Grayson, Chem. Soc. Rev. 2009, 38, 2202–2213.
- 9C. W. Bielawski, D. Benitez, R. H. Grubbs, Science 2002, 297, 2041–2044.
- 10Y. Xia, A. J. Boydston, Y. Yao, J. A. Kornfield, I. A. Gorodetskaya, H. W. Spiess, R. H. Grubbs, J. Am. Chem. Soc. 2009, 131, 2670–2677.
- 11M. L. McGraw, R. W. Clarke, E. Y. X. Chen, J. Am. Chem. Soc. 2021, 143, 3318–3322.
- 12Y. Song, J. He, Y. Zhang, R. A. Gilsdorf, E. Y. X. Chen, Nat. Chem. 2023, 15, 366–376.
- 13K.-Y. Yoon, J. Noh, Q. Gan, J. P. Edwards, R. Tuba, T.-L. Choi, R. H. Grubbs, Nat. Chem. 2022, 14, 1242–1248.
- 14Z. Miao, S. A. Gonsales, C. Ehm, F. Mentink-Vigier, C. R. Bowers, B. S. Sumerlin, A. S. Veige, Nat. Chem. 2021, 13, 792–799.
- 15Y. A. Chang, R. M. Waymouth, J. Polym. Sci. A Polym. Chem. 2017, 55, 2892–2902.
- 16H. R. Kricheldorf, J. Polym. Sci. A Polym. Chem. 2010, 48, 251–284.
- 17Y. Zhu, C. Romain, C. K. Williams, Nature 2016, 540, 354–362.
- 18R. Tong, Ind. Eng. Chem. Res. 2017, 56, 4207–4219.
- 19X. Tang, E. Y. X. Chen, Chem 2019, 5, 284–312.
- 20A. L. Chin, X. Wang, R. Tong, Macromol. Biosci. 2021, 21, 2100087.
- 21J. N. Hoskins, S. M. Grayson, Polym. Chem. 2011, 2, 289–299.
- 22P. B. Yang, M. G. Davidson, K. J. Edler, S. Brown, Biomacromolecules 2021, 22, 3649–3667.
- 23H. A. Brown, R. M. Waymouth, Acc. Chem. Res. 2013, 46, 2585–2596.
- 24H. R. Kricheldorf, S. M. Weidner, Eur. Polym. J. 2018, 105, 158–166.
- 25H. R. Kricheldorf, S. M. Weidner, A. Meyer, Polym. Chem. 2020, 11, 2182–2193.
- 26C. Hu, E. Louisy, G. Fontaine, F. Bonnet, J. Polym. Sci. A Polym. Chem. 2017, 55, 3175–3179.
- 27J. Weil, R. T. Mathers, Y. D. Y. L. Getzler, Macromolecules 2012, 45, 1118–1121.
- 28F. Bonnet, F. Stoffelbach, G. Fontaine, S. Bourbigot, RSC Adv. 2015, 5, 31303–31310.
- 29L. Zhou, L. T. Reilly, C. Shi, E. C. Quinn, E. Y. X. Chen, Nat. Chem. 2024, 16, 1357–1365.
- 30H. R. Kricheldorf, N. Lomadze, G. Schwarz, Macromolecules 2008, 41, 7812–7816.
- 31Y. Wang, T.-Q. Xu, Macromolecules 2020, 53, 8829–8836.
- 32H. R. Kricheldorf, S. M. Weidner, Polym. Chem. 2020, 11, 5249–5260.
- 33P. Piromjitpong, P. Ratanapanee, W. Thumrongpatanaraks, P. Kongsaeree, K. Phomphrai, Dalton Trans. 2012, 41, 12704.
- 34P. Wongmahasirikun, P. Prom-on, P. Sangtrirutnugul, P. Kongsaeree, K. Phomphrai, Dalton Trans. 2015, 44, 12357–12364.
- 35H. R. Kricheldorf, S. M. Weidner, F. Scheliga, Eur. Polym. J. 2019, 116, 256–264.
- 36R. W. F. Kerr, P. M. D. A. Ewing, S. K. Raman, A. D. Smith, C. K. Williams, P. L. Arnold, ACS Catal. 2021, 11, 1563–1569.
- 37C. Goonesinghe, H.-J. Jung, H. Roshandel, C. Diaz, H. A. Baalbaki, K. Nyamayaro, M. Ezhova, K. Hosseini, P. Mehrkhodavandi, ACS Catal. 2022, 12, 7677–7686.
- 38Q. Yin, L. Yin, H. Wang, J. Cheng, Acc. Chem. Res. 2015, 48, 1777–1787.
- 39X. Wang, A. L. Chin, R. Tong, Org. Mater 2021, 03, 041–050.
- 40J. Liang, T. Yin, S. Han, J. Yang, Polym. Chem. 2020, 11, 6944–6952.
- 41E. Piedra-Arroni, C. Ladavière, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2013, 135, 13306–13309.
- 42X. Wang, Z. Huo, X. Xie, N. Shanaiah, R. Tong, Chem. Asian J. 2023, 18, e202201147.
- 43X. Xie, Z. Huo, E. Jang, R. Tong, Commun. Chem. 2023, 6, 202.
- 44M. Hong, E. Y. X. Chen, Nat. Chem. 2016, 8, 42–49.
- 45J.-B. Zhu, E. M. Watson, J. Tang, E. Y.-X. Chen, Science 2018, 360, 398–403.
- 46R. Wang, J. Zhang, Q. Yin, Y. Xu, J. Cheng, R. Tong, Angew. Chem. Int. Ed. 2016, 55, 13010–13014.
- 47G. W. Coates, Chem. Rev. 2000, 100, 1223–1252.
- 48X. Wang, A. L. Chin, J. Zhou, H. Wang, R. Tong, J. Am. Chem. Soc. 2021, 143, 16813–16823.
- 49H. Wang, H. Ma, Macromolecules 2024, 57, 6156–6165.
- 50H. Li, J. Ollivier, S. M. Guillaume, J.-F. Carpentier, Angew. Chem. Int. Ed. 2022, 61, e202202386.
- 51N. Nasongkla, B. Chen, N. Macaraeg, M. E. Fox, J. M. J. Fréchet, F. C. Szoka, J. Am. Chem. Soc. 2009, 131, 3842–3843.
- 52Q. Yin, R. Tong, Y. Xu, L. W. Dobrucki, T. M. Fan, J. Cheng, Biomacromolecules 2013, 14, 920–929.
- 53O. Thillaye du Boullay, E. Marchal, B. Martin-Vaca, F. P. Cossío, D. Bourissou, J. Am. Chem. Soc. 2006, 128, 16442–16443.
- 54A. Buchard, D. R. Carbery, M. G. Davidson, P. K. Ivanova, B. J. Jeffery, G. I. Kociok-Köhn, J. P. Lowe, Angew. Chem. Int. Ed. 2014, 53, 13858–13861.
- 55Y. Lu, L. Yin, Y. Zhang, Z. Zhang, Y. Xu, R. Tong, J. Cheng, ACS Macro Lett. 2012, 1, 441–444.
- 56S. A. Schuetz, V. W. Day, R. D. Sommer, A. L. Rheingold, J. A. Belot, Inorg. Chem. 2001, 40, 5292–5295.
- 57R. Anwander, O. Runte, J. Eppinger, G. Gerstberger, E. Herdtweck, M. Spiegler, J. Chem. Soc. Dalton Trans. 1998, 847–858.
- 58S. Hong, S. Tian, M. V. Metz, T. J. Marks, J. Am. Chem. Soc. 2003, 125, 14768–14783.
- 59R. A. Andersen, K. Faegri Jr., J. C. Green, A. Haaland, M. F. Lappert, W. P. Leung, K. Rypdal, Inorg. Chem. 1988, 27, 1782–1786.
- 60D.-Y. Lee, J. F. Hartwig, Org. Lett. 2005, 7, 1169–1172.
- 61D. C. Bradley, J. S. Ghotra, F. A. Hart, J. Chem. Soc. Dalton Trans. 1973, 1021.
- 62A. R. Smith, T. Livinghouse, Organometallics 2013, 32, 1528–1530.
- 63C. A. Tolman, Chem. Rev. 1977, 77, 313–348.
- 64D. J. Durand, N. Fey, Chem. Rev. 2019, 119, 6561–6594.
- 65I. A. Guzei, M. Wendt, Dalton Trans. 2006, 3991–3999.
- 66R. Pollice, P. Chen, Angew. Chem. Int. Ed. 2019, 58, 9758–9769.
- 67X. Wang, Y. Huang, X. Xie, Y. Liu, Z. Huo, M. Lin, H. Xin, R. Tong, Nat. Commun. 2023, 14, 3647.
- 68M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, et al., Gaussian 16, Revision C.01, Gaussian, Inc. Wallingford, CT, 2016.
- 69S. Grimme, J. Antony, S. Ehrlich, H. Krieg, J. Chem. Phys. 2010, 132, 154104.
- 70J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 71C. Adamo, V. Barone, J. Chem. Phys. 1999, 110, 6158–6170.
- 72M. Ernzerhof, G. E. Scuseria, J. Chem. Phys. 1999, 110, 5029–5036.
- 73M. Dolg, H. Stoll, A. Savin, H. Preuss, Theo. Chim. Acta. 1989, 75, 173–194.
- 74L. E. Roy, P. J. Hay, R. L. Martin, J. Chem. Theory Comput. 2008, 4, 1029–1031.
- 75F. Neese, WIREs Comput. Mol. Sci. 2012, 2, 73–78.
- 76F. Neese, WIREs Comput. Mol. Sci., 2022, 12, e1606.
- 77N. Mardirossian, M. Head-Gordon, J. Chem. Phys. 2016, 144, 214110.
- 78F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065.
- 79A. V. Marenich, C. J. Cramer, D. G. Truhlar, J. Chem. Phys. B 2009, 113, 6378–6396;
- 80B. Chan, P. M. W. Gill, M. Kimura, J. Chem. Theo. Comput. 2019, 15, 3610–3622;
- 81T. Lu, Q. Chen, Comput. Theo. Chem. 2021, 1200, 113249
- 82A. P. Scott, L. Radom, J. Phys. Chem. 100, 16502–16513;
- 83C. Legault, CYLview, 1.0 b, Université de Sherbrooke, 2009.