Heteroatom Substitution Strategy Modulates Thermodynamics Towards Chemically Recyclable Polyesters and Monomeric Unit Sequence by Temperature Switching
Da Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
Search for more papers by this authorCorresponding Author
Xin Wang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
Search for more papers by this authorCorresponding Author
Zhengbiao Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, 215123 China
Search for more papers by this authorNikos Hadjichristidis
Polymer Synthesis Laboratory, KAUST Catalysis Center, Physical Sciences and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955 Saudi Arabia
Note: Prof. Nikos Hadjichristidis is an Honorary Visiting Professor at Soochow University.
Search for more papers by this authorDa Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
Search for more papers by this authorCorresponding Author
Xin Wang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
Search for more papers by this authorCorresponding Author
Zhengbiao Zhang
State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials, Jiangsu Key Laboratory of Advanced Functional Polymer Design and Application, Suzhou Key Laboratory of Macromolecular Design and Precision Synthesis, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 China
State Key Laboratory of Radiation Medicine and Protection, Soochow University, Suzhou, 215123 China
Search for more papers by this authorNikos Hadjichristidis
Polymer Synthesis Laboratory, KAUST Catalysis Center, Physical Sciences and Engineering Division, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955 Saudi Arabia
Note: Prof. Nikos Hadjichristidis is an Honorary Visiting Professor at Soochow University.
Search for more papers by this authorGraphical Abstract
Abstract
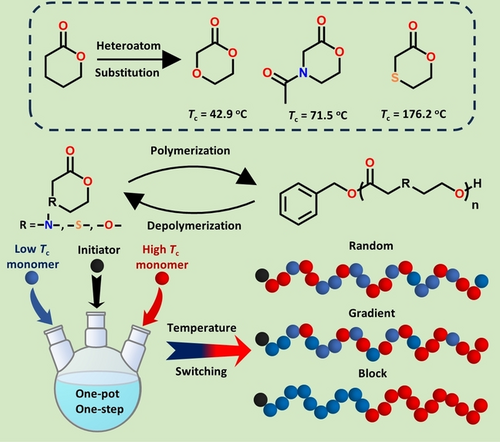
In this paper, we proposed a heteroatom substitution strategy (HSS) in the δ-valerolactone (VL) system to modulate thermodynamics toward chemically recyclable polyesters. Three VL-based monomers containing different heteroatoms (M1 (N), M2 (S), and M3 (O)), instead of C-5 carbon, were designed and synthesized to verify our proposed HSS. All three monomers undergo organocatalytic living/controlled ROP and controllable depolymerization. Impressively, the resulting P(M1) achieved over 99 % monomer recovery under both mild solution depolymerization and high vacuum pyrolysis conditions without any side reactions, and the recycled monomers can be polymerized again forming new polymers. The systematic study of the relationship between heteroatom substitution and recyclability shows that introducing heteroatoms does change the thermodynamics of the monomers (ΔHpo, ΔSpo and Tc values), thereby adjusting the polymerizability and depolymerizability. DFT calculations found that the introduction of heteroatoms adjusts the ring strain by changing the angular strain of the monomers, and the order of their angular strain (M2>M1>M3) is consistent with the order of the experimentally obtained enthalpy change. Notably, the one-pot/one-step copolymerization of two of each of the three monomers enables the synthesis of sequence-controlled copolymers from gradient to random to block structures, by simply switching the copolymerization temperature.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202402233-sup-0001-misc_information.pdf3.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. Nava, S. Chandra, J. Aherne, M. B. Alfonso, A. M. Antão-Geraldes, K. Attermeyer, R. Bao, M. Bartrons, S. A. Berger, M. Biernaczyk, R. Bissen, J. D. Brookes, D. Brown, M. Cañedo-Argüelles, M. Canle, C. Capelli, R. Carballeira, J. L. Cereijo, S. Chawchai, S. T. Christensen, K. S. Christoffersen, E. de Eyto, J. Delgado, T. N. Dornan, J. P. Doubek, J. Dusaucy, O. Erina, Z. Ersoy, H. Feuchtmayr, M. L. Frezzotti, S. Galafassi, D. Gateuille, V. Gonçalves, H.-P. Grossart, D. P. Hamilton, T. D. Harris, K. Kangur, G. B. Kankılıç, R. Kessler, C. Kiel, E. M. Krynak, À. Leiva-Presa, F. Lepori, M. G. Matias, S.-i. S Matsuzaki, Y. McElarney, B. Messyasz, M. Mitchell, M. C. Mlambo, S. N. Motitsoe, S. Nandini, V. Orlandi, C. Owens, D. Özkundakci, S. Pinnow, A. Pociecha, P. M. Raposeiro, E.-I. Rõõm, F. Rotta, N. Salmaso, S. S. S. Sarma, D. Sartirana, F. Scordo, C. Sibomana, D. Siewert, K. Stepanowska, Ü. N. Tavşanoğlu, M. Tereshina, J. Thompson, M. Tolotti, A. Valois, P. Verburg, B. Welsh, B. Wesolek, G. A. Weyhenmeyer, N. Wu, E. Zawisza, L. Zink, B. Leoni, Nature 2023, 619, 317–322;
- 1bH. T. Pinheiro, C. MacDonald, R. G. Santos, R. Ali, A. Bobat, B. J. Cresswell, R. Francini-Filho, R. Freitas, G. F. Galbraith, P. Musembi, T. A. Phelps, J. P. Quimbayo, T. E. A. L. Quiros, B. Shepherd, P. V. Stefanoudis, S. Talma, J. B. Teixeira, L. C. Woodall, L. A. Rocha, Nature 2023, 619, 311–316;
- 1cJ. Smith, S. Vignieri, Science 2021, 373, 34–35.
- 2
- 2aZ. O. G. Schyns, M. P. Shaver, Macromol. Rapid Commun. 2020, 42, 2000415;
- 2bC. N. Waters, J. Zalasiewicz, C. Summerhayes, A. D. Barnosky, C. Poirier, A. Gałuszka, A. Cearreta, M. Edgeworth, E. C. Ellis, M. Ellis, C. Jeandel, R. Leinfelder, J. R. McNeill, D. d Richter, W. Steffen, J. Syvitski, D. Vidas, M. Wagreich, M. Williams, A. Zhisheng, J. Grinevald, E. Odada, N. Oreskes, A. P. Wolfe, Science 2016, 351, aad2622;
- 2cW.-T. Hsu, T. Domenech, W. McDowall, Clean. Environ. Syst. 2021, 2, 100004.
- 3
- 3aJ.-B. Zhu, E. M. Watson, J. Tang, E. Y.-X. Chen, Science 2018, 360, 398–403;
- 3bG. W. Coates, Y. D. Y. L. Getzler, Nat. Rev. Mater. 2020, 5, 501–516.
- 4
- 4aJ. B. Zhu, E. Y. Chen, Angew. Chem. Int. Ed. 2018, 57, 12558–12562;
- 4bY.-T. Guo, C. Shi, T.-Y. Du, X.-Y. Cheng, F.-S. Du, Z.-C. Li, Macromolecules 2022, 55, 4000–4010.
- 5R. M. Cywar, N. A. Rorrer, H. B. Mayes, A. K. Maurya, C. J. Tassone, G. T. Beckham, E. Y. Chen, J. Am. Chem. Soc. 2022, 144, 5366–5376.
- 6
- 6aJ. Payne, M. D. Jones, ChemSusChem 2021, 14, 4041–4070;
- 6bD. E. Fagnani, J. L. Tami, G. Copley, M. N. Clemons, Y. Getzler, A. J. McNeil, ACS Macro Lett. 2021, 10, 41–53.
- 7
- 7aQ. Song, C. Pascouau, J. Zhao, G. Zhang, F. Peruch, S. Carlotti, Prog. Polym. Sci. 2020, 110, 101309;
- 7bG. Xu, Q. Wang, Green Chem. 2022, 24, 2321–2346.
- 8C. Shi, L. T. Reilly, V. S. Phani Kumar, M. W. Coile, S. R. Nicholson, L. J. Broadbelt, G. T. Beckham, E. Y. X. Chen, Chem 2021, 7, 2896–2912.
- 9C. M. Plummer, L. Li, Y. Chen, Macromolecules 2023, 56, 731–750.
- 10
- 10aX. L. Li, K. Ma, F. Xu, T. Q. Xu, Chem. Asian J. 2023, 18, e202201167;
- 10bX. B. Lu, Y. Liu, H. Zhou, Chem. Eur. J. 2018, 24, 11255–11266.
- 11
- 11aC. Shi, Z.-C. Li, L. Caporaso, L. Cavallo, L. Falivene, E. Y. X. Chen, Chem 2021, 7, 670–685;
- 11bJ. Yuan, W. Xiong, X. Zhou, Y. Zhang, D. Shi, Z. Li, H. Lu, J. Am. Chem. Soc. 2019, 141, 4928–4935;
- 11cC. Shi, M. L. McGraw, Z.-C. Li, L. Cavallo, L. Falivene, E. Y.-X. Chen, Sci. Adv. 2020, 6, eabc0495;
- 11dY. M. Tu, X. M. Wang, X. Yang, H. Z. Fan, F. L. Gong, Z. Cai, J. B. Zhu, J. Am. Chem. Soc. 2021, 143, 20591–20597;
- 11eJ. Bruckmoser, S. Remke, B. Rieger, ACS Macro Lett. 2022, 11, 1162–1166.
- 12
- 12aY. M. Tu, F. L. Gong, Y. C. Wu, Z. Cai, J. B. Zhu, Nat. Commun. 2023, 14, 3198;
- 12bX. L. Li, R. W. Clarke, J. Y. Jiang, T. Q. Xu, E. Y. Chen, Nat. Chem. 2023, 15, 278–285;
- 12cY. Shen, W. Xiong, Y. Li, Z. Zhao, H. Lu, Z. Li, CCS Chem. 2021, 3, 620–630;
- 12dL. Zhou, Z. Zhang, C. Shi, M. Scoti, D. K. Barange, R. R. Gowda, E. Y.-X. Chen, Science 2023, 380, 64–69;
- 12eC. Li, L. Wang, Q. Yan, F. Liu, Y. Shen, Z. Li, Angew. Chem. Int. Ed. 2022, 61, e202201407;
- 12fJ. Li, F. Liu, Y. Liu, Y. Shen, Z. Li, Angew. Chem. Int. Ed. 2022, 61, e202207105.
- 13
- 13aY. Wang, M. Li, J. Chen, Y. Tao, X. Wang, Angew. Chem. Int. Ed. 2021, 60, 22547–22553;
- 13bY. Wang, Y. Zhu, W. Lv, X. Wang, Y. Tao, J. Am. Chem. Soc. 2023, 145, 1877–1885;
- 13cK. A. Stellmach, M. K. Paul, M. Xu, Y. L. Su, L. Fu, A. R. Toland, H. Tran, L. Chen, R. Ramprasad, W. R. Gutekunst, ACS Macro Lett. 2022, 11, 895–901;
- 13dL.-G. Li, Q.-Y. Wang, Q.-Y. Zheng, F.-S. Du, Z.-C. Li, Macromolecules 2021, 54, 6745–6752;
- 13eC.-Y. Lyu, W. Xiong, E.-Q. Chen, H. Lu, Chin. J. Polym. Sci. 2023, 41, 1555–1562;
- 13fJ.-Z. Zhao, T.-J. Yue, B.-H. Ren, Y. Liu, W.-M. Ren, X.-B. Lu, Macromolecules 2022, 55, 8651–8658.
- 14M. Wang, Z. Ding, X. Shi, Z. Ma, B. Wang, Y. Li, Macromolecules 2024, 57, 869–879.
- 15
- 15aN. R. Conley, L. A. Labios, D. M. Pearson, C. C. L. McCrory, R. M. Waymouth, Organometallics 2007, 26, 5447–5453;
- 15bG.-J. t Brink, I. W. C. E. Arends, M. Hoogenraad, G. Verspui, R. A. Sheldon, Adv. Synth. Catal. 2003, 345, 1341–1352.
- 16Q. Y. Wang, M. X. Cao, X. W. Kan, A. Lv, F. S. Du, Z. C. Li, J. Polym. Sci. 2022, 60, 1976–1987.
- 17B. G. G. Lohmeijer, R. C. Pratt, F. Leibfarth, J. W. Logan, D. A. Long, A. P. Dove, F. Nederberg, J. Choi, C. Wade, R. M. Waymouth, J. L. Hedrick, Macromolecules 2006, 39, 8574–8583.
- 18R. C. Pratt, B. G. G. Lohmeijer, D. A. Long, R. M. Waymouth, J. L. Hedrick, J. Am. Chem. Soc. 2006, 128, 4556–4557.
- 19S. Naumann, P. B. V. Scholten, J. A. Wilson, A. P. Dove, J. Am. Chem. Soc. 2015, 137, 14439–14445.
- 20X. Wang, N. Hadjichristidis, ACS Macro Lett. 2020, 9, 464–470.
- 21C. Alemán, O. Betran, J. Casanovas, K. N. Houk, H. K. Hall Jr., J. Org. Chem. 2009, 74, 6237–6244.
- 22
- 22aT. R. Blake, R. M. Waymouth, J. Am. Chem. Soc. 2014, 136, 9252–9255;
- 22bC. Aleman, O. Betran, J. Casanovas, K. N. Houk, H. K. Hall Jr., J. Org. Chem. 2009, 74, 6237–6244.
- 23P. Olsén, K. Odelius, A.-C. Albertsson, Biomacromolecules 2016, 17, 699–709.
- 24
- 24aG.-Q. Tian, Z.-H. Yang, W. Zhang, S.-C. Chen, L. Chen, G. Wu, Y.-Z. Wang, Green Chem. 2022, 24, 4490–4497;
- 24bW. Zhang, G.-Q. Tian, G. Wu, S.-C. Chen, Y.-Z. Wang, Green Chem. 2023, 25, 5517–5525.
- 25T. Gleede, J. C. Markwart, N. Huber, E. Rieger, F. R. Wurm, Macromolecules 2019, 52, 9703–9714.
- 26Y. Yu, G. Storti, M. Morbidelli, Ind. Eng. Chem. Res. 2011, 50, 7927–7940.