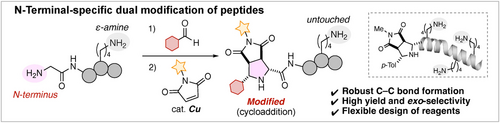
N-Terminal-Specific Dual Modification of Peptides through Copper-Catalyzed [3+2] Cycloaddition
Graphical Abstract
We developed a method for the N-terminal-specific dual modification of peptides through a three-component [3+2] cycloaddition with aldehydes and maleimides under mild copper catalysis. This approach enables exclusive functionalization at the glycine N-terminus of peptides, regardless of the presence of lysine ϵ-amine, affording the chemically robust pyrrolidine ring in excellent yields with complete exo-diastereoselectivity.
Abstract
Site-specific introduction of multiple components into peptides is greatly needed for the preparation of densely functionalized and structurally uniform peptides. In this regard, N-terminal-specific peptide modification is attractive, but it can be difficult due to the presence of highly nucleophilic lysine ϵ-amine. In this work, we developed a method for the N-terminal-specific dual modification of peptides through a three-component [3+2] cycloaddition with aldehydes and maleimides under mild copper catalysis. This approach enables exclusive functionalization at the glycine N-terminus of iminopeptides, regardless of the presence of lysine ϵ-amine, thus affording the cycloadducts in excellent yields. Tolerating a broad range of functional groups and molecules, the present method provides the opportunity to rapidly construct doubly functionalized peptides using readily accessible aldehyde and maleimide modules.
Introduction
Peptides and peptidomimetics are essential as therapeutic agents, drug candidates, chemical biology probes, and biomaterials.1 Consequently, the development of methods for late-stage peptide modification and conjugation is becoming increasingly crucial for diversifying peptide structures, modulating drug pharmacokinetics, and introducing functional moieties, such as fluorophores, for molecular imaging.2 Nevertheless, the selective modification of peptides to produce their structurally uniform conjugates remains challenging due to the presence of various nucleophilic sites, particularly the highly reactive and abundant lysine ϵ-amino group, among other sites (Figure 1A).3, 4
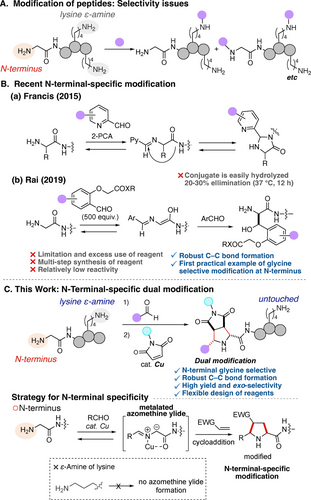
Approaches to N-terminal-selective peptide modification. (A) Issues of peptide modification. (B) Recent N-terminal-specific modification. (C) This work: N-Terminal-specific dual modification.
In recent years, the selective modification of the N-terminus of peptides has received significant attention for two primary reasons.5, 6 First, the inherent uniqueness of the N-terminus, among other functional groups, makes it an attractive and specific target for single-site chemical modification. Second, modification of the N-terminus, rather than the functional group(s) within the peptide backbone, is believed to have a reduced influence on the three-dimensional structure, and hence on the function, of the original peptide. The N-terminal-selective modification has been achieved through the involvement of associated amino acid side chains (e.g., Cys, Ser, and Thr)5 or condensation with ketenes or aldehydes under carefully controlled pH conditions.7
Besides these strategies, the use of well-designed aldehyde reagents that allow N-terminal-specific functionalization through the unique reactivity of the transiently formed imines has been successful.6 Francis et al.6a reported an N-terminal-selective modification using pyridine-2-carboxaldehyde-based reagents, which involves the imine formation, followed by cyclization to an imidazolidinone ring (Figure 1B(a)). While this method has found broad applications in life sciences research,8 its main drawback resides in the equilibrium elimination of the introduced aldehyde moiety under typical physiological conditions. In 2019, Rai et al. identified carboxylic acid- or amide-appended aldehydes that can convert the glycine N-terminus into amino alcohol moieties through imine formation, equilibrium generation of enol species, and its aldol reaction with a second molecule of the aldehyde (Figure 1B(b)).6b Although this method enables irreversible modification of the glycine N-terminus through robust C−C bond formation, the aldehyde reagents must be synthesized in multiple steps and used in large excess quantities to install the functional molecules of interest.
In addition to site selectivity, the simultaneous installation of multiple distinct functional molecules also represents a current challenge in peptide modification9 to meet the growing demand for the combined use of drug and imaging agents as well as multimodality imaging for advanced biological studies.10, 11 The aforementioned N-terminal-selective modifications are inadequate for this challenge because such bimolecular reactions only allow for the installation of one functional molecule at a time. Therefore, the three-component and site-selective modification of peptides using two readily available reaction components, each of which can accommodate a distinct functional molecule, is highly desirable. However, to the best of our knowledge, such modular and flexible peptide modifications at the N-terminus cannot be found in the literature.9
Herein, we describe a methodology for performing dual modifications at the glycine N-terminus of peptides. This is achieved through the implementation of a copper-catalyzed three-component [3+2] cycloaddition reaction involving aldehydes and maleimides, leading to the formation of robust C−C bonds (Figure 1C). This approach capitalizes on the exclusive and efficient generation of metalated azomethine ylides,12, 13, 14, 15 a pivotal catalytic intermediate for the cycloaddition process, from the imine formed at the N-terminus even in the presence of various functional groups from the peptide itself as well as from the aldehyde and the maleimide. As a testament to the versatility and robustness of this design strategy, our method facilitates the precise and efficient modification of the N-termini of various oligo- and polypeptides, accommodating sequences containing up to 26 amino acid residues and multiple lysine moieties. As such, the present work has significantly extended the chemical space accessible via the [3+2] cycloaddition of metalated azomethine ylides, a domain hitherto largely confined in the context of small molecule synthesis from simple amino acid esters12, 16 and some amino acid amides.15 Given the broad functional group compatibility and the ready accessibility of the aldehyde and maleimide modules, the present three-component functionalization provides a versatile and high yielding means to install two distinct functional molecules into peptides in a simultaneous and divergent manner.9, 10, 11
Results and Discussion
At the outset of the present study, we explored the reaction conditions for the catalytic 1,3-dipolar cycloaddition of preformed imino-dipeptide 1 a derived from H-Gly-Gly-OMe (R1=p-Tol) and N-methylmaleimide (2 a) (Figure 2A; see Table S1 for the optimization details). A catalyst generated from Cu(MeCN)4PF6 and Xantphos was found to promote the [3+2] cycloaddition reaction to afford the desired [3+2] cycloadduct 3 a exclusively as an exo-diastereomer.17 Specifically, stirring a mixture of 1 a (1.0 equiv.), 2 a (1.1 equiv.), Cu(MeCN)4PF6 (5 mol %), Xantphos (5.5 mol %), and Et3N (20 mol %) in MeCN at room temperature for 16 h afforded the fused pyrrolidine 3 a in a quantitative yield. This reaction could be performed in a variety of solvents, including polar (MeCN, acetone, DMSO, and DMF), ethereal (THF), and halogenated (CH2Cl2) solvents, affording the product quantitatively. Furthermore, the reaction proceeded efficiently even when water was added (88 % yield in MeCN/H2O 95 : 5 (v/v)). Silver–phosphine catalysts also promoted the cycloaddition but produced a mixture of diastereomers. Note that the LiBr-based conditions employed in the [3+2] cycloaddition of macrocyclic azomethine ylide16c failed to promote the present reaction.
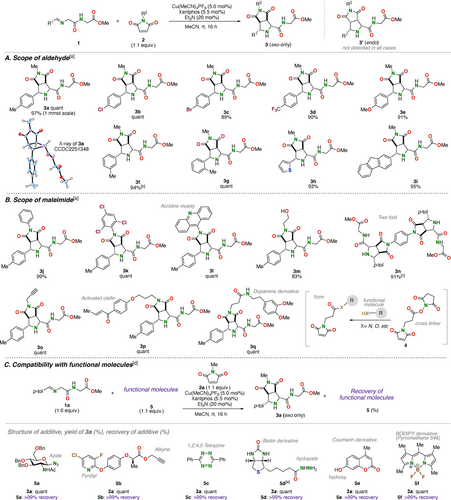
1,3-Dipolar cycloaddition reaction using functionalized iminopeptides 1 and maleimides 2. (A) Scope of the iminopeptides 1. (B) Scope of the maleimides 2. (C) Compatibility with functional molecules 5. [a] The isolated yields are shown. [b] 30 mol % of Et3N was used. [c] 2.1 equiv. of iminopeptide was used. [d] The yields were determined by NMR analysis. [e] DMF was used as the solvent.
The optimized conditions for the Cu/Xantphos-catalyzed [3+2] cycloaddition were successfully applied to a broad range of substituted imino-dipeptides 1 and maleimides 2 (Figure 2A and B). Iminopeptides 1 derived from electronically and sterically diverse (hetero)aromatic aldehydes underwent a smooth cycloaddition with N-methylmaleimide (2 a), affording the desired products 3 b–3 i in high to excellent yields with exclusive exo-selectivity (Figure 2A). These reactions tolerated various functional groups, including synthetically versatile halogens (3 b, 3 c), trifluoromethyl (3 d), ether (3 e), thiophene (3 h), and fluorene (3 i). Meanwhile, various functionalized maleimides were demonstrated to participate in the reaction with iminopeptide 1 a (Figure 2B). N-(Het)aryl- or hydroxyethyl-substituted maleimides were quantitatively coupled with 1 a to afford the corresponding fused-pyrrolidines 3 j–3 m, again as single diastereomers. Notably, the acridine fluorophore-substituted maleimide was well tolerated (3 l). The use of a phenylene-linked bismaleimide as a dipolarophile led to a two-fold cycloaddition to produce 3 n in an excellent yield. A terminal alkyne-substituted maleimide also produced the cycloadduct 3 o quantitatively, despite potential interference from competing copper acetylide formation. This product was amenable to further functionalization by the CuAAC (Cu-Catalyzed Azide Alkyne Cycloaddition) reaction with 3′-azido-3′-deoxythymidine (Figure S1).18 A maleimide substrate containing an additional enone moiety underwent chemoselective cycloaddition on the maleimide moiety to afford the cycloadduct 3 p quantitatively, leaving the enone moiety intact for further transformation. Furthermore, an amide-containing maleimide, prepared from crosslinking reagent 4 and a dopamine derivative, afforded the desired cycloadduct 3 q in a quantitative yield. This result demonstrates that readily available crosslinking reagents containing N-hydroxysuccinimide (NHS) ester and maleimide moieties offer a means to install amine- and alcohol-based modules into the peptide.
Having established the basic scope of iminopeptides and maleimides, we became interested in further investigation into the robustness of the present [3+2] cycloaddition to the influence of various functional groups and molecules.19 To quickly address this issue, the model reaction between 1 a and 2 a was performed in the presence of a variety of functional molecules that are frequently used as tools for chemical biology (Figure 2C). For example, the reaction proceeded smoothly in the presence of a glucopyranose derivative bearing azide and acetamide (5 a), a diaryl ether bearing a pyridyl group and a terminal alkyne (5 b), 3,6-diphenyl-1,2,4,5-tetrazine (5 c), and a biotin derivative bearing hydrazide (5 d), all of which contain functional group handles for click reactions. Thus, the reaction invariably afforded the cycloadduct quantitatively with complete diastereoselectivity, leaving the added molecule untouched. Interestingly, the hydrazide moiety of 5 d, which could easily undergo transimination with the iminopeptide 1 a, did not interfere with the desired reaction. Likewise, fluorescent molecules such as a hydroxycoumarin derivative 5 e and a BODIPY derivative 5 f did not adversely affect the reaction.
Next, a series of control experiments were performed to probe the selectivity of the present [3+2] cycloaddition toward the glycine N-terminus (Figure 3). A competitive reaction using an equimolar mixture of iminopeptides 1 a and 1 j, derived from H-Gly-Gly-OMe and H-l-Lys-Gly-OMe, respectively, with maleimide 2 a (1.1 equiv.) as the coupling partner, exclusively and quantitatively afforded the cycloadduct of the former (3 a), with the latter being almost (97 %) recovered (Figure 3A). Thus, not only did 1 j remain intact toward [3+2] cycloaddition at its more hindered N-terminal, but it also did not cause any interference with the reaction of 1 a by its ϵ-amino-derived imine. Furthermore, iminopeptide 1 k prepared from H-Gly-l-Lys-OMe efficiently reacted with 2 a only at the N-terminus to afford the cycloadduct 3 r in 85 % yield after transimination of the unreacted extra imine moiety by treatment with MeONH2 ⋅ HCl (Figure 3B).
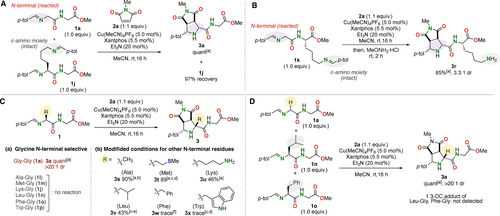
(A, B) N-Terminal selectivity of the [3+2] cycloaddition. (C) N-Terminal glycine selectivity and modified conditions for other N-terminal residues. (D) N-Terminal glycine-selective modification in a mixture of iminopeptides. The yields were determined by 1H NMR analysis unless otherwise noted. [a] The isolated yields are shown. [b] Iminopeptide 1 (1.1 equiv.) and Et3N (3.0 equiv.) were used. [c] Maleimide 2 (3.0 equiv.) was used. [d] Cu(MeCN)4PF6 (20 mol %), Xantphos (22 mol %), and Et3N (10 equiv.) were used. [e] Reaction was conducted at 50 °C. [f] Iminopeptide 1 (2.0 equiv.) and Et3N (6.0 equiv.) were used.
The N-terminal glycine selectivity of the present method was further explored. As N-Terminal glycine is readily exposed by recombinant expression,20 N-terminal glycine selective modification is promising for universal methodology in life sciences research. In addition, glycine-selective reactions are expected to be scarce and valuable methods for glycine-selective labeling and purification from mixtures of several peptide terminals.6b However, only a few examples of N-terminal glycine-selective reactions have been reported6b, 21 due to the difficulty of recognizing the glycine moiety that lacks an assisting side chain.22 Iminopeptides having N-terminal Ala, Met, Lys, Leu, Phe, and Trp residues did not undergo [3+2] cycloaddition with 2 a under the standard conditions, which stands in contrast to the quantitative transformation of the N-terminal glycine of 1 a (Figure 3C(a)). Meanwhile, a simple modification of the standard conditions, i.e., an increased loading of Et3N (3 equiv. instead of 20 mol %), allowed us to engage the iminopeptide derived from H-l-Ala-Gly-OMe in the cycloaddition with 2 a to give product 3 s in excellent yield and exclusive diastereoselectivity (Figure 3C(b)). Although the reactivity of the peptides decreased with increasing bulk of the substituent, additional catalyst loading and heating promoted the transformation of the N-terminal Met, Lys, and Leu moieties to afford 3 t–3 v. This result holds promise for the extension of the present approach to general N-terminal-selective peptide modifications. The high N-terminal glycine-selectivity of the standard conditions was also demonstrated by an intermolecular competition using a mixture of imines bearing N-terminal Gly, Leu, and Phe residues, which afforded the cycloadduct 3 a as the sole product in quantitative yield (Figure 3D). According to these results, the present method is considered promising for the selective purification or labeling of N-terminal glycine peptides through the introduction of appropriate purification tags or labeling molecules.
Given the attractiveness of three-component assembly methods for the expedient construction of densely functionalized peptides, including those containing multimodality imaging probes,9, 10, 11 we moved on to a one-pot reaction between peptides, aldehydes, and maleimides (Figure 4). To our delight, a sequence of the imine formation from glycylglycine methyl ester (6 a) and 4-methylbenzaldehyde (7 a) and the Cu-catalyzed cycloaddition with 2 a proceeded efficiently in a one-pot manner to afford the cycloadduct 3 a in quantitative yield (Figure 4A). This protocol could be applied to various aldehydes, including alkylaldehydes (e.g., cyclohexanecarboxaldehyde), to afford the desired cycloadducts in excellent yields. Meanwhile, various di- and tripeptides were also demonstrated to participate in the reaction with 4-methylbenzaldehyde (7 a) to afford the desired cycloadducts 3 aa–3 ad in excellent yields. Besides a series of di- and tripeptides containing H-Gly-Gly moiety (3 aa–3 ac), the peptide containing a substituted amino acid (Phe) next to the N-terminus also afforded the desired product 3 ad in high yield. However, the reaction using H-Gly-l-Ala-OMe was somewhat sluggish and gave the cycloadduct 3 ae in 60 % yield owing to the instability of the free base peptide.
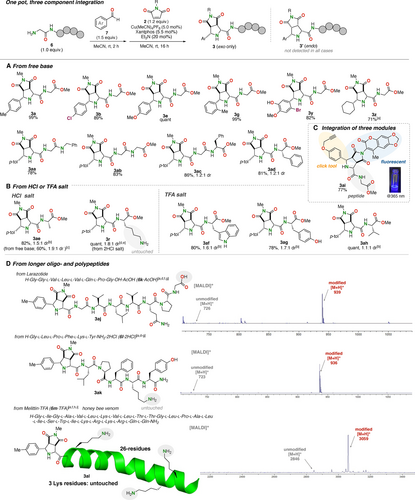
One-pot, three-component integration. (A) The one-pot reaction using free base peptides. (B) The one-pot reaction using peptide salts. (C) Demonstration of one-pot integration of three components. (D) Modification of longer oligo- and polypeptides. The isolated yields are shown. [a] Cu(MeCN)4PF6 (10 mol %) and Xantphos (11 mol %) were used. [b] Et3N (1st step: 1.0 equiv., 2nd step: 5.0 equiv.) was used. [c] From free base peptide without using additional Et3N. [d] Et3N (1st step: 2.0 equiv., 2nd step: 10 equiv.) was used. [e] The reaction mixture was treated with MeONH2 ⋅ HCl in MeOH to remove the imine moiety. [f] DMF was used as the solvent. [g] Maleimide (1.1 equiv.) was used. [h] Maleimide (1.0 equiv.), aldehyde (6.0 equiv.), Cu(MeCN)4PF6 (20 mol %), Xantphos (22 mol %), and Et3N (1st step: 4.0 equiv., 2nd step: 20 equiv.) were used. [i] PDBe: 2mlt.
This problem could be resolved by using the stable HCl salt of the peptide in combination with an extra amount of Et3N. With the modified protocol, the reaction proceeded smoothly to afford 3 ae in a significantly improved yield of 82 % (Figure 4B). Furthermore, the one-pot reaction was also feasible with the HCl salt of lysine containing peptide (H-Gly-l-Lys-OMe ⋅ 2HCl), affording the desired cycloadduct 3 r in quantitative yields with complete N-terminal selectivity. Furthermore, the TFA salts of Gly-l-Trp-OMe, Gly-l-Tyr-OMe, and Gly-l-Val-OMe successfully afforded the cycloadducts 3 af–3 ah in high yields. It should be noted that the free base of H-Gly-l-Lys-OMe is unstable and cannot be isolated in a pure form. Thus, the modified protocol using HCl/TFA salts would be useful not only because free base peptides are often unstable but also because peptides are often cut out in the form of salts after solid-phase synthesis.
The one-pot protocol is both time-efficient and practically more viable as it obviates preformed iminopeptides, which are often nontrivial to isolate in pure form. Moreover, this method enables rapid and simultaneous installation of two functional molecules into a peptide.9 As a demonstration of this point, a clickable aldehyde and a fluorophore-containing maleimide could be introduced into the peptide 6 a in a one-pot manner to afford the bifunctional peptide 3 ai in high yield (Figure 4C). The promise of the one-pot protocol was further demonstrated by the rapid three-component integration of longer oligopeptides and polypeptides (Figure 4D). Thus, the C-terminal unprotected substrate 6 k ⋅ AcOH (Larazotide acetate) cleanly gave the three-component conjugates 3 aj despite the presence of the CO2H moiety, as indicated by the MALDI-MS analysis. The heptapeptide 6 l ⋅ 2HCl was selectively modified at the N-terminus to afford the single conjugate 3 ak with very high efficiency. To our delight, this reaction was further applied to a longer polypeptide, Melittin ⋅ TFA (6 m ⋅ TFA; honeybee venom). The reaction of 6 m ⋅ TFA proceeded quite efficiently to produce the cycloadduct 3 al, leaving three lysine ϵ-amine moieties untouched. These results collectively demonstrate the utility of the present one-pot assembly method using peptide salts for the uniform, site-specific, and efficient bifunctionalization of oligopeptides.
Conclusion
In summary, we have developed a method for the glycine N-terminal-selective dual modification of peptides through a 1,3-dipolar cycloaddition reaction with the formation of robust C−C bonds. The present method capitalizes on the site-specific generation of an azomethine ylide at the N-terminus of iminopeptides and its highly efficient [3+2] cycloaddition with maleimides under copper catalysis. The cycloaddition takes place under mild conditions and affords the cycloadducts in excellent yields with complete exo-diastereoselectivity across a variety of preformed and in situ-generated iminopeptides as well as functionalized maleimides while tolerating various functional groups and functional molecules, including the ϵ-amine of a lysine residue. In particular, the one-pot three-component protocol allows the straightforward assembly of diverse di-, tri-, and oligopeptides with aldehydes and maleimides, thus offering an opportunity for the convenient and expeditious construction of doubly functionalized peptides. We anticipate that the present method and its further improvement will enable single-site dual modification of more complex and bioactive peptides and proteins using a diverse set of functional molecule-tagged aldehydes and maleimides. Further studies along this line are currently underway.
Supporting Information
The Supporting Information is available free of charge. Experimental procedures; characterization for new compounds including NMR spectra (PDF).
Acknowledgments
We thank Prof. Naohiko Yoshikai (Tohoku University), Prof. Shin-ichi Fukuzawa (Chuo University), and Prof. Haruhiko Fuwa (Chuo University) for the fruitful discussions. We thank Mr. Shohei Furuya for the X-ray analysis. This study was supported by the Kato Memorial Bioscience Foundation (K. K.), the Tokyo Biochemical Research Foundation (K. K.), the Takahashi Industrial and Economic Research Foundation (K. K.), a Meiji Seika Award in Synthetic Organic Chemistry, Japan (K. K.), The Uehara Memorial Foundation (K. K.), the NOVARTIS Foundation (Japan) for the Promotion of Science (K. K.), the Research Foundation for Pharmaceutical Sciences (K. K.), the Institute of Science and Engineering, Chuo University, and JSPS KAKENHI (Grant Numbers, JP20K15288 and JP22K14687: Young Scientists; K. K.).
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.