Light-Triggered Disassembly of Molecular Motor-based Supramolecular Polymers Revealed by High-Speed AFM
Graphical Abstract
Abstract
Photoresponsive supramolecular polymers have a major potential for applications in responsive materials that are externally triggered by light with spatio-temporal control of their polymerisation state. While changes in macroscopic properties revealed the adaptive nature of these materials, it remains challenging to capture the dynamic depolymerisation process at the molecular level, which requires fast observation techniques combined with in situ irradiation. By implementing in situ UV illumination into a High-Speed Atomic Force Microscope (HS-AFM) setup, we have been able to capture the disassembly of a light-driven molecular motor-based supramolecular polymer. The real-time visualisation of the light-triggered disassembly process not only reveals cooperative depolymerisation, it also shows that this process continues after illumination is halted. Combining the data with cryo-electron microscopy and spectroscopy approaches, we obtain a molecular-level description of the motor-based polymer dynamics reminiscent of actin chain-end depolymerisation. Our detailed understanding of supramolecular depolymerisation will drive the development of future responsive polymer systems.
Introduction
Depolymerisation of biomolecular polymers is often essential to perform specific cellular functions, such as the separation of chromosomes during mitosis by the depolymerisation of microtubules1 and the control of cell motility and morphogenesis by actin filaments.2 Also for synthetic polymers, dynamic (de)polymerisation might prove crucial, particularly in bio-inspired approaches to achieve responsive functions in molecular systems as well as control of assembly-disassembly processes along length scales. Synthetic, supramolecular polymers consist of small, functional building blocks that self-assemble into well-defined, elongated structures through non-covalent interactions.3 They are of interest in a variety of scientific fields including optoelectronics, sensing, and self-healing materials.4, 5 Moreover, supramolecular polymers can be designed biocompatible, which makes them interesting for potential biomedical applications such as tissue engineering and drug delivery.6, 7 To control property and function of the polymers, different stimuli have been introduced.4, 8-10 One of them is light, which is a powerful stimulus able to manipulate systems with high spatial and temporal accuracy. By incorporating light-sensitive molecules, such as azobenzene or spiropyran units, materials gain the ability to change their structure and properties upon irradiation with light.11, 12
Synthetic molecular motors have attracted increasing attention due to their potential application in various fields, enabling the transformation from molecules to dynamic molecular systems.13-19 In comparison to conventional two-state photoswitches, light-driven molecular rotary motors can undergo unidirectional motions, featuring sequential photoisomerisation and thermal helix inversion (THI) processes enabling to drive systems away from equilibrium states.20 They have been successfully applied in polymer systems enabling macroscopic motion, multistate emission and chirality transfer.10, 21-28 The inherent chirality of the molecular motor can be transferred to supramolecular polymers,29 opening up the possibility to control supramolecular chirality. While the polymerisation of self-assembling supramolecular polymers has been extensively studied,30-32 the direct observation of photo-induced supramolecular depolymerisation process remains challenging.33 This is in particular the case for aqueous media which are crucial for biomedical applications. In addition, understanding the dynamic disassembly process is vital for designing a variety of functional materials that contain a depolymerisation step. Light-induced disassembly has been studied ex situ by scanning electron microscopy,34 transmission electron microscopy35, 36 and atomic force microscopy.34, 37 In these ex situ experiments, only the polymers before and after depolymerisation have been imaged. However, the dynamic process of disassembly itself has not been captured. Direct observation of the disassembly kinetics is challenging due to the limited dimensions and because it requires microscopy with in situ irradiation. Fluorescence microscopy is capable of capturing light-induced disruption of assembled structures in real-time,38 but lacks the lateral resolution to observe the kinetics of depolymerisation of polymers at the nanometre scale.
The emergence of High-Speed Atomic Force Microscopy (HS-AFM)39 allows for label-less, real-time observation of nanoscale dynamic processes with sub-second temporal resolution. Therefore, HS-AFM is well suited to study polymer dynamics. The technique has up-to-now predominantly been used to study dynamic biophysical processes,40-46 yet has also been applied to observe self-assembly47 and repair48 of synthetic supramolecular polymers. By combining HS-AFM with in situ irradiation, real-time observation of the structural response of light-driven molecular motor-based dynamics becomes possible. Understanding which rotational state of a motor affects supramolecular polymer depolymerisation is particularly of interest for the design and application of molecular motor-based responsive systems.
Here, we present the first direct observation of the light-driven depolymerisation of a molecular motor-based supramolecular polymer with intrinsic chirality. HS-AFM data demonstrate that depolymerisation only occurs at the polymer ends, reminiscent of actin depolymerisation. Strikingly, depolymerisation even continues after the light has been turned off. This discovery integrally shows how light can trigger a cooperative process of depolymerisation capable of sustaining itself after the light trigger is absent.
Results and Discussion
Structural Characterisation of Molecular Motor-Based Supramolecular Polymers
In our previous study, the molecular motor-based bis-urea derivative (R,R)-(P,P)-cis-MUOEG6 has been demonstrated to form helical supramolecular polymers in aqueous solution.29 The cycle of the process of the motor is fourfold, consisting of two photoisomerisation steps, each followed by THI. Upon photoisomerisation of stable (R,R)-(P,P)-cis-MUOEG6, metastable (R,R)-(M,M)-trans-MUOEG6 is formed, which converts to stable (R,R)-(P,P)-trans-MUOEG6 during THI (see Scheme S2B). Further illumination results in photochemical conversion to metastable (R,R)-(M,M)-cis-MUOEG6 after which stable (R,R)-(P,P)-cis-MUOEG6 is recovered after THI. In this system, structural changes upon isomerisation result in the destabilisation of the polymer structure. To investigate whether subtle structural changes of the molecular motor can affect the assembly structure and disassembly dynamics of motor-based supramolecular polymers, two new compounds were synthesised: (S,S)-(M,M)-cis-MUOEG6 (the enantiomer of (R,R)-(P,P)-cis-MUOEG6) and a version with shorter polyethylene glycol sidechain length, (S,S)-(M,M)-cis-MUOEG4, aiming to reduce the hydrophilicity (see Scheme 1A). Details of the synthesis procedure are summarised in the Supporting Information (Scheme S1). All new compounds have been characterised by 1H & 13C nuclear magnetic resonance (NMR) and high-resolution mass spectrometry (HRMS) (see Figures S20–S31).
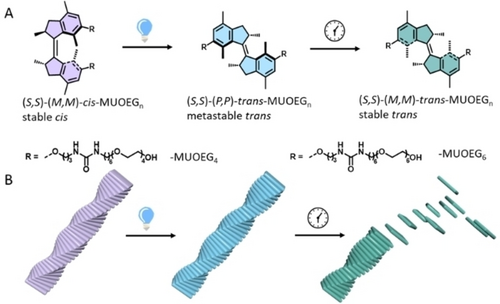
Molecular structures and multi-state rotation of molecular motors. (A) Schematic structure of stable (S,S)-(M,M)-cis-MUOEGn isomer, the metastable (S,S)-(P,P)-trans-MUOEGn state which is formed upon radiation by 300 nm light for 3 min and the following stable (S,S)-(M,M)-trans-MUOEGn state after THI over a timespan of ~20 min in the dark. (B) Schematic depiction of the fiber in each state.
To investigate the assembled structure, stable (S,S)-(M,M)-cis-MUOEG4 based supramolecular polymers were immobilised on a silicon-dioxide substrate and imaged by AFM (see Video S1, Figure S5). AFM images of (S,S)-(M,M)-cis-MUOEG4 show helical fibers (see Figures 1A–B) exhibiting a periodic variation in height with minima and maxima of 1.1±0.2 nm and 2.0±0.2 nm respectively. The half pitch distance of the helical turn is 98±33 nm. The diameter of (S,S)-(M,M)-cis-MUOEG4 is comparable to measurements of (S,S)-(M,M)-cis-MUOEG6 and (R,R)-(P,P)-cis-MUOEG6 (see Figure S1–S3, table S1), which suggests that the diastereomers also form fibers by one-dimensional stacking of the molecular motor-based monomers.29 The helical half-pitch distance of (S,S)-(M,M)-cis-MUOEG4 supramolecular polymers is slightly larger than those formed by (S,S)-(M,M)-cis-MUOEG6.
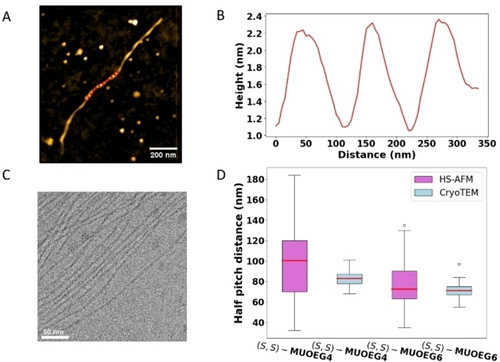
Structure of molecular motor-based supramolecular polymers. (A) AFM image of supramolecular polymer formed by (S,S)-(M,M)-cis-MUOEG4 in water deposited on a thermal oxide silicon substrate. (B) Cross section along the dashed red line in (A), showing the helical periodicity. (C) Cryo-TEM image of supramolecular polymers formed by (S,S)-(M,M)-cis-MUOEG4. (D) Box plot showing the half pitch of supramolecular polymers formed by (S,S)-(M,M)-cis-MUOEG4 and (S,S)-(M,M)-cis-MUOEG6, measured by HS-AFM (purple) and Cryo-TEM (blue). The red line indicates the mean, the boxes the interquartile regions (IQR) and the whiskers the upper and lower bounds of 1.5 * IQR. Any outliers are indicated with circles.
For further structural analysis, Cryo-TEM imaging was performed (see Figure 1C–D, S13, Table S2). The maximum width of the fiber of (S,S)-(M,M)-cis-MUOEG4 is measured to be 7.8±0.9 nm, the narrow part is 3.8±0.5 nm and the helical pitch was measured to be 82±5 nm (see Figure S15). The measured diameter varies between HS-AFM and Cryo-TEM results. We contribute this to the extension of the hydrophilic tails in solution, whereas on a surface those will most likely be compressed as a result of the surface immobilisation and the transient contact during HS-AFM. Cryo-TEM images of fibers of (S,S)-(M,M)-cis-MUOEG6 reveal comparable diameters (see Figure S14) as for (S,S)-(M,M)-cis-MUOEG4 with a slightly lower averaged half-pitch value of 75±4 nm, consistent with the trend found in AFM. The dimensions of the fibers of (S,S)-(M,M)-cis-MUOEG4 and MUOEG6 are in good agreement with the previously reported size of (R,R)-(P,P)-cis-MUOEG6.29
Rotation of Molecular Motors in Supramolecular Polymers
To investigate the chirality transfer from the molecular motor to the handedness of the supramolecular polymers, circular dichroism (CD) spectroscopy measurements were performed. The CD spectrum of supramolecular polymer assembled of (S,S)-(M,M)-cis-MUOEG4 shows a characteristic absorption band and negative Cotton effect on at 270–360 nm (see Figure 2A and 2D). The CD spectra of supramolecular polymers of (S,S)-(M,M)-cis-MUOEG4 and MUOEG6 are close to the mirror image of that of (R,R)-(P,P)-cis-MUOEG6 supramolecular polymers, which showed a positive Cotton effect, suggesting the opposite supramolecular chirality of the fibers. An aqueous solution of the supramolecular polymer formed by (S,S)-(M,M)-cis-MUOEG4 was irradiated with 300 nm light. Upon irradiation, the absorption band was generated at 360–400 nm with a decrease of the band at 280–340 nm, suggesting the formation of a metastable trans isomer (S,S)-(P,P)-trans-MUOEG4 (see Figure 2A). After irradiating for 3 min, the photostationary state (PSS) was reached. Now the CD spectrum exhibits a negative CD signal at 360–400 nm due to the newly generated absorption band (see Figure 2D). Subsequent storage of the sample in darkness for 20 min at room temperature resulted in a decrease in the absorption band at 330–400 nm and an increase in the band at 260–310 nm, showing the THI of metastable trans to stable trans isomer (S,S)-(M,M)-trans-MUOEG4 (see Figure 2B). The negative CD signal at 330–400 nm disappeared after 15 min of THI at room temperature, indicating the full conversion of the metastable trans isomer to stable trans isomer (see Figure 2D). The isomerisation process of (S,S)-(M,M)-cis-MUOEG4 is comparable to that of (R,R)-(P,P)-cis-MUOEG6.29 Eyring analysis on the THI of metastable trans MUOEG4 reveals a half-life time of 2.8 min, and an energy barrier (≠G°) of 85.1 kJ mol−1 (see Figure 2C, Table S3). Molecular motors with longer hydrophilic chains, (S,S)-(M,M)-cis-MUOEG6, were characterised using the same method and they show similar spectra (see Figures S17–19). Eyring analysis on the THI of metastable trans MUOEG6 showed a half-life time of 2.9 min and an energy barrier (≠G°) of 85.2 kJ mol−1, which is comparable to the result of MUOEG4.
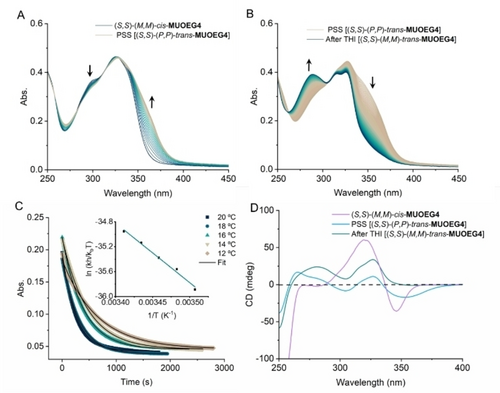
UV/Vis absorption and CD spectra of the supramolecular polymer assembled of molecular motors in water. (A) UV/Vis absorption spectra of stable (S,S)-(M,M)-cis-MUOEG4 40 μM in water upon 300 nm light irradiation for 3 min to get metastable (S,S)-(P,P)-trans-MUOEG4 at 20 °C, (B) after subsequently keeping in the dark at 20 °C for 20 min to reach stable (S,S)-(M,M)-trans-MUOEG4. (C) Time-dependent absorption changes at 365 nm during the THI of metastable (S,S)-(P,P)-trans-MUOEG4 in water at different temperatures. Insert: Eyring analysis on the THI of (S,S)-(P,P)-trans-MUOEG4 in water. (D) CD spectra of stable (S,S)-(M,M)-cis-MUOEG4 (60 μM in water) (purple curve) upon 300 nm light irradiation for 3 min to get metastable (S,S)-(P,P)-trans-MUOEG4 (blue curve) and subsequent keeping in the dark for 15 min to reach stable (S,S)-(M,M)-trans-MUOEG4 (cyan curve) at room temperature.
In Situ Observation of Depolymerisation Upon Irradiation by HS-AFM
In order to capture the photoinduced dynamics of molecular motor-based systems in real-time, we implemented a UV source into our HS-AFM set-up (see Figure 3A). Stable (S,S)-(M,M)-cis-MUOEG4 supramolecular polymers were continuously imaged before and during irradiation. Figure 3B–C (Video S2) displays the polymer response to 300 nm-UV light. Interestingly, upon irradiation, we observed the reduction of the polymer from the free ends only. The depolymerisation of a single fiber shows clear phases of slow/stagnated and rapid depolymerisation with varying durations. No correlation was found between the depolymerisation speed and the position of the fiber edge with respect to the helical turn (see Figure S13). Some fibers started depolymerising directly upon irradiation, whereas others showed lag phases of up to 150s to initialise depolymerisation (see Figure S12B). The average depolymerisation speed of all measured (S,S)-(M,M)-cis-MUOEG4 fibers is 1.0±0.8 nm s−1, varying from 0.1 nm s−1 to 2.7 nm s−1, and is independent of the imaging rate (see Figure S12A) At both edges molecules detached from the fiber structure, whereas no disruption of the structure in the middle of the fiber was visible during irradiation (see Figure 3D and Figure S6). No significant difference in average depolymerisation velocity was observed between the two sides of the fiber (0.8±0.4 vs 1.0±0.5 nm s−1 for the slower side vs the faster side of each measured fiber). To exclude the effect of the tip-sample interaction we imaged (S,S)-(M,M)-cis-MUOEG4, and retracted the tip during irradiation. After 15 min we imaged the same position again and indeed the fibers had been depolymerising from the ends during this period as a result of the illumination only (see Figure S7). The spherical aggregates on the background do not show clear signs of photoresponsivity and do not appear to exchange material with the fibers (see Figure S11). Depolymerisation from the fiber ends has additionally been observed for (S,S)-(M,M)-cis-MUOEG6 and (P,P)-(R,R)-cis-MUOEG6 (see Figure S9–S10, Video S3–4), with similar characteristics (see Figure 3E).
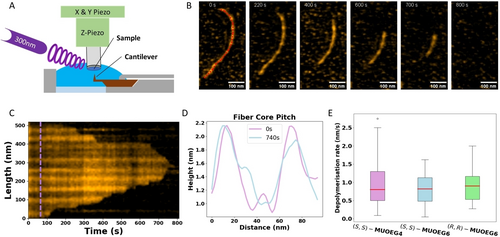
Direct visualisation of photo-induced depolymerisation process by HS-AFM. (A) Schematic representation of the HS-AFM setup with in situ irradiation. (B) Real time depolymerisation of (S,S)-(M,M)-cis-MUOEG4 upon irradiation by 300 nm light from 60s onwards. (C) Time resolved intensity kymograph taken along the red dashed line in (B), the bright horizontal stripes indicate the fiber pitch. The position at which the UV was turned on is indicated by the purple dashed line. (D) Cross section of the fiber centre at 0 s and 740s, showing the preservation of the helical pitch in the fiber centre during depolymerisation (E) Box plot showing the depolymerisation rates for the three fiber types with n=17, 9 and 23 for (S,S)-(M,M)-cis-MUOEG4, (S,S)-(M,M)-cis-MUOEG6 and (R,R)-(P,P)-cis-MUOEG6 respectively. The red line indicates the mean, the boxes the interquartile regions (IQR) and the whiskers the upper and lower bounds of 1.5 * IQR. Any outliers are indicated with circles.
Interestingly, there appeared to be a delay between the moment of irradiation and the onset of depolymerisation of supramolecular polymers (see Figure S12B). This suggests depolymerisation does not immediately follow from photoinduced isomerisation of the stable cis to metastable trans motor, yet might depend on the THI of the molecular motor. The depolymerisation speed of the three molecules is comparable (see Figure 3E), revealing that the depolymerisation process is conserved over these various molecular motor-based supramolecular polymers.
Our observations reveal that molecules at both free ends detach upon irradiation, while the structure in the fiber centre remains unaltered. From our UV/Vis experiments, we observed that upon irradiation PSS was reached within 3 min, and subsequent 20 min in the dark were necessary for full conversion through THI to the stable trans state (see Figure 2). In accordance with our previous findings,29 the half-life of metastable (R,R)-(M,M)-trans-MUOEG6 increased in aqueous solution where the molecules are stabilised in the fibrillar structure, and we report similar effects for (S,S)-(M,M)-cis-MUOEG4 and (S,S)-(M,M)-cis-MUOEG6.
The variability of detachment rates at the fiber ends indicates that molecules are not free to undergo THI immediately after the detachment of their direct neighbour. Crowding around photoswitchable units has been shown to have a large effect on the isomerisation behaviour and transition rate in supramolecular polymers49 and in our system, the steric hindrance of neighbouring molecules could have similar effects. Aggregation-Induced-Emission (AIE) has been observed before for (R,R)-(P,P)-MUOEG6 upon irradiation and attributed to the restriction of the excited-state rotation of the molecular motor in confined space.29 Therefore, we hypothesise the variance in the detachment rates to be the result of the time-dependent THI process that is necessary to destabilise the fiber structure, while molecules that still have two neighbouring molecules are tightly packed and thus sterically hindered in their movement. The kinetic behaviour of the enantiomers demonstrated that the depolymerisation process does not depend on the chirality of the molecular motor core. Similar molecular motors designed with a shorter hydrophilic sidechain to decrease hydrophilicity and increase the hydrophobic effect, showed comparable disassembly kinetics as observed by HS-AFM. This result is consistent with Eyring analysis of the THI of the molecular motors in aqueous supramolecular polymers, in which the energy barriers of the THI and half-life times are comparable for all designed molecules. All three molecules are equipped with bis-urea moieties to enhance the stability of the polymeric form. This further supports our hypothesis that the limiting factor of depolymerisation is mainly the THI of molecular motors and the corresponding cleavage of bis-urea hydrogen bonds during the rotation of the molecular motor.
In the case of amphiphilic structures, light-activation resulted in fast and random destabilisation through photochemically induced disassembly.38 In our systems, with direct supramolecular bonds constraining the molecular motor core, the depolymerisation occurred with precise control only at the ends of the structures. These differences indicate again that the initial supramolecular polymer structure and resulting packaging and crowding of the molecular motor play an important role in which motors are available for rotation and for disrupting the structure. Designing photoresponsive molecules that form tightly packed fibrillar structures is a step towards controlling supramolecular polymer function by irradiation.
In Situ Observation of Depolymerisation After Halting Irradiation
To further support our observation that the THI process might be responsible for the depolymerisation of the polymer, we set out to investigate the effect of brief irradiation on the polymer structure of (S,S)-(M,M)-cis-MUOEG4. Polymers of (S,S)-(M,M)-cis-MUOEG4 were prepared on a silicon dioxide substrate as described above. Upon irradiation, disassembly occurred from both ends of the fiber, yet unexpectedly, we found that upon switching the LED off, the fiber continued to depolymerise (see Figure 4, Video S5). Interestingly, there was no clear change in depolymerisation speed after switching the LED off (0.3±0.5 vs 0.3±0.9 nm/s). Upon irradiation of the polymer, metastable trans isomers (S,S)-(P,P)-trans-MUOEG4 are formed immediately, whereas the depolymerisation of fibers was delayed by about 3 min, which is close to the time required for reaching PSS. After removal of the UV light the fiber continued to depolymerise, indicating that the depolymerisation occurs during the THI step to stable (S,S)-(M,M)-trans-MUOEG4. To distinguish whether the depolymerisation after stopping the light irradiation was spontaneous or due to disturbance forces, irradiation and imaging were halted at the same time. After 10 min the same location was imaged and indicated complete depolymerisation of the fibers (see Figure S8). This is in good agreement with UV/Vis experiments, where the photoisomerisation from stable cis to metastable trans takes around 3 min to reach PSS and subsequent THI of metastable trans takes 20 min to completely convert to the stable trans-state in the dark. Thus, even without new photons arriving, the polymer continued to be disrupted at the free ends. This shows that an initial trigger by irradiating the polymers is enough to set the cooperative disassembly process in motion and that no additional light is needed. The origin of this remarkable finding could be that the rotation of a monomer at the fiber end, and as a consequence subsequent monomer detachment, creates enough space for the neighbouring molecule to undergo a conformational change. Further studies on the detailed mechanics are ongoing.
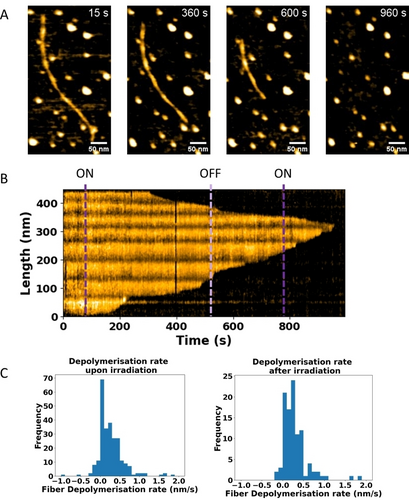
The depolymerisation rate is unaffected by halting irradiation. (A) Snapshots of (S,S)-(M,M)-cis-MUOEG4 depolymerisation upon irradiation from 60s to 525 s, subsequently in the dark from 525 s to 780s and finally irradiated again. (B) Time resolved intensity kymograph along the fiber. Positions where the UV was turned on and off are indicated by magenta and purple lines respectively. (C) Histogram of depolymerisation speeds measured every 5 sec upon (left, 0.3±0.5 nm/s) and after (right, 0.3±0.9 nm/s) irradiation.
Conclusion
To summarise, we presented the direct observation of photo-induced depolymerisation of molecular motor-based supramolecular polymers in real-time at the nanoscale with HS-AFM using in situ irradiation. By photo-actively triggering selective depolymerisation of only the ends of supramolecular polymers we are able to mimic some of nature's specifically designed processes that are dependent on depolymerisation.1, 2 Using chiral molecules, we have shown that we can transfer the chirality of the motors to the supramolecular polymer, while maintaining similar kinetic behaviour. This paves the way for designing functions based on homochirality, another quality biomolecular polymers often possess.50 Outstanding features of the studied molecular motor-based polymers are that the depolymerisation process is cooperative and continues at the same pace after illumination is halted. This unique feature allows for applicability in environments where continuous irradiation would harm other processes. Our results allow for a deeper understanding of the photo-induced depolymerisation process of supramolecular polymers and highlights the potential of HS-AFM in the field of molecular motors and responsive supramolecular polymers.
Acknowledgments
WHR is supported by a Dieptestrategie grant of the Zernike Institute National Research Centre of the Rijksuniversiteit Groningen. BLF acknowledges the Ministry of Education, Culture and Science of the Netherlands (Gravitation Program No. 024.001.035 to B.L.F.)
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.