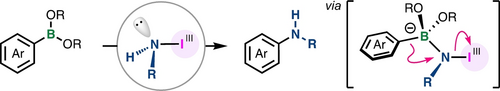
Amino-λ3-iodane-Enabled Electrophilic Amination of Arylboronic Acid Derivatives
Graphical Abstract
Amino-λ3-iodanes enable the electrophilic amination of arylboronic acids and boronates. Iodine(III) reagents with transferable amino groups, including one with an NH2 group, were synthesized and used in the amination, allowing the synthesis of a wide range of primary and secondary (hetero)arylamines.
Abstract
In this report, we describe the use of amino-λ3-iodanes in the electrophilic amination of arylboronic acids and boronates. Iodine(III) reagents with transferable amino groups, including one with an NH2 group, were synthesized and used in the amination, allowing the synthesis of a wide range of primary and secondary (hetero)arylamines. Mechanistic studies by DFT calculations indicate that the reaction proceeds through an electrophilic amination process from a tetravalent borate complex with a B−N dative bond.
The development of methodologies for the synthesis of arylamines is a research topic of great interest in organic synthesis as they are important building blocks that are ubiquitous in natural products, pharmaceuticals, agrochemicals, and organic materials.1 The most fundamental approach to accessing arylamines is C(aryl)−N bond-forming reactions. Classical methods include nucleophilic aromatic substitution (SNAr) with amines2 and electrophilic nitration of aromatic compounds.3 Despite their reliability, these reactions often suffer from low levels of functional group compatibility due to the harsh reaction conditions. Over the past few decades, transition-metal (TM)-catalyzed C(aryl)−N bond-forming reactions such as Buchwald–Hartwig4 and Ullmann-Goldberg-type5 coupling have been the most widely used examples. In addition, electrophilic amination of organometallic reagents6 and C(aryl)−H amination7 have also found wide application in arylamine synthesis. The use of TMs, however, has a serious drawback, particularly in the production of pharmaceuticals when trace metal contaminants must be completely removed, which is a laborious process.8 In this context, practical TM-free methods are in high demand.
Electrophilic amination of arylboron reagents has attracted considerable attention—particularly the use of readily available and easily handled boronic acid derivatives.9 In recent decades, TM-free approaches have been developed using various electrophilic aminating reagents such as hydroxyamine derivatives,10 an aminoazanium,11 azides,12 and others13, 14 (Scheme 1) as alternatives to TM-catalyzed reactions (e.g. Chan–Lam-Evans coupling).15 Existing methods, however, continue to offer only limited applicability in reactions involving electron-deficient (hetero)arylboronic acids and pinacol boronates. In addition, the examples of synthesized secondary amines remain scarce.10c, 10e, 14 The electrophilic amination of arylboron reagents generally proceeds via the formation of a tetravalent borate complex with a B−N dative bond that is followed by 1,2-aryl migration, and, therefore, an aminating reagent that possesses both nucleo- and electrophilic characteristics is required. We envisioned that amino-λ3-iodanes containing a nucleophilic amino functionality attached to the iodine(III) center with high leaving ability16 would qualify as an aminating reagent for such amination.17 Although there are several examples of defined hypervalent iodine reagents containing transferable nitrogen functional groups,18 their nitrogen atoms generally possess electron-withdrawing groups that reduce their nucleophilicity. Recently, iodine(III) reagents containing aliphatic amino groups such as secondary amines19a and benzylic primary amines19b have been synthesized. However, the scope of amines is still limited, especially an NH2-substituted one has never been reported. To date, there are no examples of the electrophilic amination of arylboron reagents utilizing hypervalent iodine-based aminating reagents. Herein, we report the syntheses of various amino-λ3-iodanes, which includes one with an NH2 group, and describe their use in the electrophilic amination of arylboronic acids and boronates (Scheme 1). The amination developed in this study provides a powerful tool for the synthesis of primary and secondary (hetero)arylamines, which are difficult to access using existing methods.
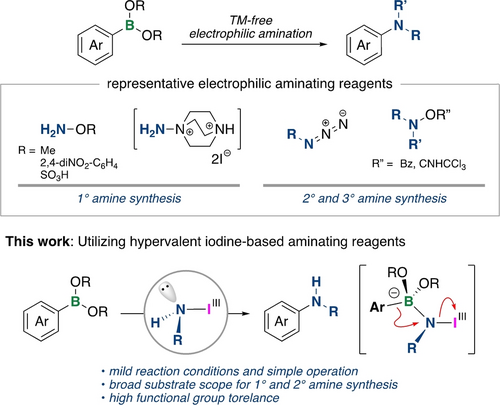
Transition-metal-free electrophilic amination of arylboronic acid derivatives.
We initially investigated the synthesis of an NH2-substituted hypervalent iodine reagent, which is a promising reagent for the direct synthesis of primary amines but has never been synthesized. An iodine reagent having a benziodoxolone skeleton was selected in consideration of the stability of the target compound.18b, 20 A ligand exchange of the acetoxy group of 1-acetoxy-1,2-benzodioxol-3-(1H)-one (2) with commercially available hexamethyldisilazane (R=H, 3 a) as an ammonia surrogate was then examined, which resulted in a low yield of 1 a with the formation of unidentified inseparable byproducts. The addition of EtOH (1.5 equiv) as a proton source was found to be effective in suppressing the byproduct formation and improving the yield of 1 a up to 92 % in a 10 mmol scale synthesis (Scheme 2). Single crystals of 1 a suitable for X-ray crystallographic analysis were grown via slow diffusion from an acetonitrile solution.21 The structural data clearly confirmed that the sp3-hybridized NH2 group is bound to the iodine center with an I−N bond length of 2.025(6) Å (Figure 1), which approximates the sum of the covalent radii of N and I (2.10 Å).22 The single-bond characteristics of the I−N bond were also supported by Wiberg bond index (WBI) analysis (WBI(N−I)=0.789) (vide infra). In the crystal packing, the 1 a forms intermolecular secondary bonding interactions that include hydrogen bonding between the NH2 group and the oxygen atoms and also halogen bonding between the iodine atom and the oxygen atom (see SI, Figure S1). Furthermore, various disilazanes 3, readily prepared from primary amines,23 could be used in the ligand exchange process to synthesize derivatives containing allyl (1 d), propargyl (1 e), siloxy (1 f), and sterically hindered cyclohexyl (1 g) groups. This simple method would be a promising tool for the preparation of new types of hypervalent iodine reagents containing transferable amino groups. The synthesized reagents 1 are reasonably stable under ambient conditions in the solid state.24 Differential scanning calorimetry (DSC) analysis was performed to check the stability of 1 a. The DSC result showed that the decomposition started at 130 °C with a heat release of 572 J/g (see SI, Figure S2).
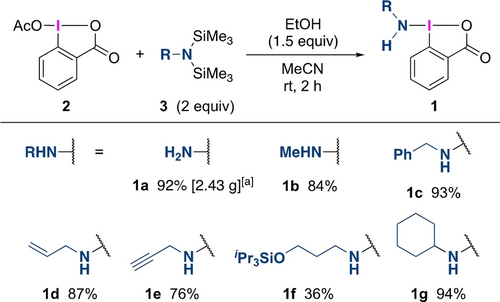
Synthesis of amino-λ3-iodanes. Yields are isolated yields. [a] The reaction was conducted on a 10 mmol scale.
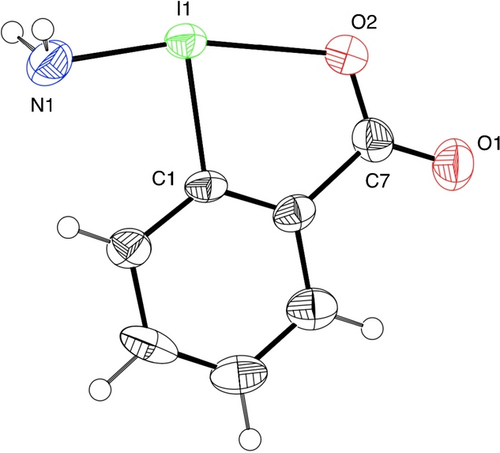
Crystal structure of 1 a. Thermal ellipsoids are shown at the 50 % probability level. Selected bond lengths [Å] and angles [deg]: I1−N1 2.025(6), I1−O2 2.385(5), I1−C1 2.113(5), N1−I1−O2 166.59(18); N1−I1−C1 92.4(2), 74.29(18), C7−O2−I1 114.3(4).
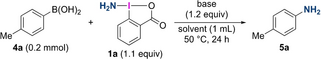
We then turned our attention to the electrophilic amination of arylboronic acids using synthesized amino-λ3-iodane 1 a to provide a primary amine (Table 1). The reaction of 4-methylphenylboronic acid (4 a) with 1 a in THF at 50 °C resulted in the formation of 5 a in a low yield (entry 1). Screening of the reaction conditions revealed that the addition of a base is effective in promoting amination (entries 2–5), and the use of Cs2CO3 drastically improved the yield of 5 a up to 94 % (entry 4). The amination also proceeded at room temperature with slightly lower efficiency (entry 6). Reducing the amount of Cs2CO3 resulted in a lower yield of 5 a (entry 7). Examining the reaction in other solvents showed that THF is suitable for this amination (entries 8–11).
|
|||
Entry |
Base |
Solvent |
Yield [%][a] |
|---|---|---|---|
1 |
none |
THF |
9 |
2 |
Na2CO3 |
THF |
18 |
3 |
K2CO3 |
THF |
79 |
4[b] |
Cs2CO3 |
THF |
94 (84) |
5 |
NEt3 |
THF |
48 |
6[c] |
Cs2CO3 |
THF |
70 |
7[d] |
Cs2CO3 |
THF |
65 |
8 |
Cs2CO3 |
1,4-Dioxane |
87 |
9 |
Cs2CO3 |
MeCN |
65 |
10 |
Cs2CO3 |
Toluene |
70 |
11 |
Cs2CO3 |
DMSO |
26 |
- [a] Determined by 1H NMR analysis of the crude product. A value in parenthesis is an isolated yield. [b] The reaction was conducted on a 0.4 mmol scale. [c] The reaction was conducted at room temperature. [d] Cs2CO3 (0.6 equiv) was used.
With the optimized reaction conditions in hand, we explored the scope of the amination of arylboronic acids and their derivatives using 1 a (Scheme 3). A number of arylboronic acids bearing both electron-donating and -withdrawing groups on the aryl rings were efficiently aminated. Remarkably, highly electron-deficient substrates, which have generally shown low reactivity in previously reported methods3, 10, 11 were transformed into the corresponding anilines in high yields (5 n–5 x).25 Various functional groups including heterocycle (5 c, 5 j–5 l), hydroxy (5 d, 5 e), trimethylsilyl (5 g), alkene (5 h), alkyne (5 i), nitro (5 n, 5 u), sulfonyl (5 o), several types of carbonyl (5 p–5 r, 5 v, 5 x), and nitrile (5 s, 5 w) moieties are well tolerated. The sterically demanding anilines 5 y and 5 z could also be synthesized. It is noteworthy that although the amination of heteroarylboronic acids is difficult to achieve using the existing methods,10, 11 the present method was successfully applied to the amination of substrates containing indole (5 ab), pyridine (5 ac), pyrimidine (5 ad), (iso)quinoline (5 ae–5 ag), benzofuran (5 ah), thiophene (5 ai), isoxazole (5 aj), and pyrazole (5 ak, 5 al) skeletons. The synthetic utility of this amination was further demonstrated by the synthesis of the loratadine derivative 5 am via a C−H borylation/electrophilic amination sequence. Although the reported methods of the amination of less-reactive pinacol boronic acid esters usually require the use of strong bases,10a, 11 they readily underwent amination under the mild reaction conditions (5 i, 5 l, 5 u, 5 x, 5 ag, 5 ah, 5 aj, 5 ak, 5 al, 5 am), ensuring the synthetic usefulness of the present method. A gram-scale reaction afforded 5 q without loss of efficiency, which demonstrates the scalability of the reaction. In addition, boroxine 6 and (triol)borate salt 7 were also suitable substrates, and, interestingly, the latter case required no addition of Cs2CO3. Meanwhile, trifluoroborate salt 8 did not undergo the amination. It is also found that the present method can be applied to the amination of an alkylboronic acid (see the Supporting Information). Furthermore, the use of reagents 1 allowed the direct synthesis of secondary amines. The reaction of boronic acid 4 a with 1 b under the standard reaction conditions resulted in a low yield of 5 an along with some amounts of p-cresol as a byproduct, but the addition of molecular sieves was effective in suppressing the side reaction and improving the yield of 5 an up to 80 %. By employing the modified reaction conditions, secondary amines 5 an–5 av were successfully synthesized in moderate to good yields.26
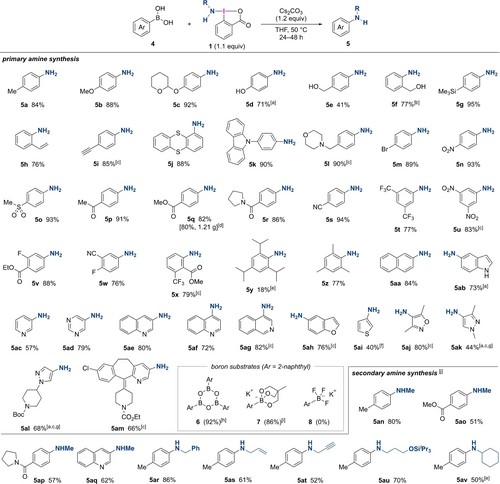
Substrate scope. Reactions were conducted on a 0.2–0.4 mmol scale. Yields are isolated yields. Yields in parentheses were determined by 1H NMR analysis of the crude product. [a] The reaction was conducted at room temperature. [b] Benzo[c][1,2]oxaborol-1(3H)-ol was used as a substrate. [c] Pinacol boronic acid esters were used as a substrate. [d] The reaction was conducted on a 10 mmol scale. [e] The reaction was conducted at 70 °C. [f] Yield of the isolated product after Boc protection. [g] 1 a (1.5 equiv) and Cs2CO3 (1.5 equiv) were used. [h] 6 (0.33 equiv) was used. [i] The reaction was conducted in the absence of Cs2CO3. [j] MS3 A (200–800 mg) was added. Boc=tert-butoxycarbonyl.
Based on the previous studies on the electrophilic amination of boronic acids,10, 11-13, 14 we proposed that the reaction would be initiated by the formation of a tetravalent borate complex through a B−N dative bond, which would then be followed by 1,2-aryl migration. To investigate the mechanistic details of the present electrophilic amination, density functional theory (DFT) calculations were performed at the M06-2X/6-311++g(d,p)-SDD(I,Cs),SMD(THF)//M06-2X/6-31+g(d,p)-LANL2DZ(I,Cs),SMD(THF) level of theory using phenylboronic acid as a model substrate, and the resultant energy profile is shown in Figure 2a. The Natural bond orbital (NBO) analysis was first performed to understand the electronic characteristics of 1 a (Figure 2b). The NBO charge of the sp3-hybridized nitrogen atom of 1 a was calculated to be −1.16, which supports the presumption of its nucleophilic nature. The coordination of the amino group of 1 a to the boron center of PhB(OH)2 to form INT1, was determined to be an endothermic process (8.9 kcal/mol).10b, 10d The 1,2-aryl migration from INT1 via TS1′ has a large free-energy barrier of 39.1 kcal/mol relative to the separated PhB(OH)2 and 1 a, which is consistent with experimental results showing low efficiency for amination in the absence of a base. Meanwhile, in the presence of Cs2CO3, both the abstraction of the proton at the nitrogen atom and the coordination of the carboxyl group to the cesium cation occur to form the thermodynamically more stable INT2 (see SI, Figure S3). The 1,2-aryl migration from this complex via TS1 has a much lower free energy barrier (12.0 kcal/mol) than that of TS1′, which led to the formations of 9 and 10. It is suggested that Cs2CO3 acts as a base, and the resulting cesium cation then acts as a Lewis acid to promote the elimination of ortho-iodobenzoate, which then allows an efficient 1,2-aryl migration. The WBI and calculated bond lengths in TS1 clearly show that cleavages of the C−B bond (WBI(C−B)=0.564, d=1.72 Å) and the N−I bond (WBI(N−I)=0.536, d=2.42 Å) occur simultaneously with the formation of the Ph−N bond (WBI(C−N)=0.303, d=2.07 Å), which suggests a concerted 1,2-aryl migration mechanism (Figure 2b and c). Furthermore, NBO analysis of TS1 revealed that there is a strong σ-donation interaction (81.6 kcal/mol) between the bonding orbital of σ(C−B) and the antibonding orbital of σ*(N−I), which supports the electrophilic amination mechanism (see SI, Figure S4). The decrease in the NBO charge on the iodine atom from 1.25 in INT2 to 0.66 in TS1 indicates the reduction of I(III) to I(I). To gain insight into the nature of the TS1, a Hammett study was performed using a series of para-substituted arylboronic acids (Scheme 4). More electron-rich substrates showed higher reactivity, and a linear correlation between the log([5-Ar]/[5-Ph]) and Hammett constants (σpara) was observed with a negative slope (ρ=−0.84, R2=0.99),27 indicating that a buildup in the positive charge at the aryl group occurs in TS1. This result is consistent with calculation showing that the positive charge of the aryl group, particularly at the ipso carbon, increases from −0.30 in INT2 to −0.19 in TS1. The amination of a pinacol boronate with 1 a also proceeds efficiently. Indeed, DFT calculations of the reaction pathway were performed, and the resultant reaction energy profile was similar to that of the reaction of a boronic acid (see SI, Figure S5).
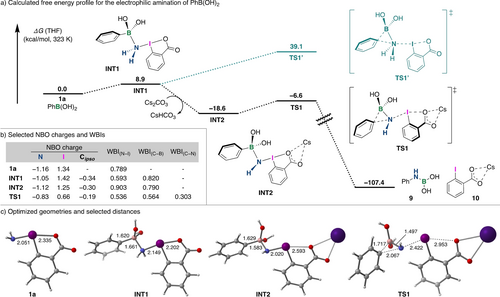
DFT calculation studies. Free energies (kcal/mol) are computed at the M06-2X/6–311++g(d,p)-SDD(I,Cs),SMD(THF)//M06-2X/6–31+g(d,p)-LANL2DZ(I,Cs),SMD(THF) level of theory.
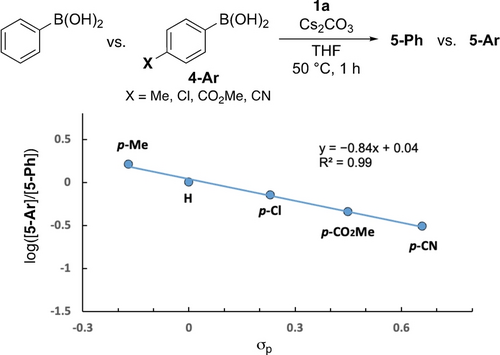
Hammett analysis.
In conclusion, we have developed an electrophilic amination of arylboronic acids and boronates utilizing newly synthesized amino-λ3-iodanes that exhibit both nucleo- and electrophilic reactivity. The addition of Cs2CO3 is key to the promotion of the reaction, and the Cs-mediated electrophilic amination mechanism was supported by DFT calculations. This reaction has a broad substrate scope with good functional group tolerance. Thus, we anticipate that the method will find wide applications in organic synthesis.
Acknowledgments
This work was supported by JSPS KAKENHI Grant Numbers JP22H02078 and JP22H05362, and JST, the establishment of university fellowships towards the creation of science technology innovation, Grant Number JPMJFS2125. K.K. also thanks the research fund of the Asahi Glass Foundation and the Uehara Memorial Foundation.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.