Controlling Monoterpene Isomerization by Guiding Challenging Carbocation Rearrangement Reactions in Engineered Squalene-Hopene Cyclases
Graphical Abstract
Carbocation-based rearrangements are vital for terpene versatility. AacSHC triterpene cyclase from Alicyclobacillus acidocaldarius acted as a promiscuous catalyst for multiple monoterpene rearrangements. Tailored AacSHC variants directed the specific isomerization of pinene with exceptional selectivities. Success involved restructuring the aromatic active pocket and reorganizing water clusters to stabilize demanding carbocationic intermediates.
Abstract
The interconversion of monoterpenes is facilitated by a complex network of carbocation rearrangement pathways. Controlling these isomerization pathways is challenging when using common Brønsted and Lewis acid catalysts, which often produce product mixtures that are difficult to separate. In contrast, natural monoterpene cyclases exhibit high control over the carbocation rearrangement reactions but are reliant on phosphorylated substrates. In this study, we present engineered squalene-hopene cyclases from Alicyclobacillus acidocaldarius (AacSHC) that catalyze the challenging isomerization of monoterpenes with unprecedented precision. Starting from a promiscuous isomerization of (+)-β-pinene, we first demonstrate noticeable shifts in the product distribution solely by introducing single point mutations. Furthermore, we showcase the tuneable cation steering by enhancing (+)-borneol selectivity from 1 % to >90 % (>99 % de) aided by iterative saturation mutagenesis. Our combined experimental and computational data suggest that the reorganization of key aromatic residues leads to the restructuring of the water network that facilitates the selective termination of the secondary isobornyl cation. This work expands our mechanistic understanding of carbocation rearrangements and sets the stage for target-oriented skeletal reorganization of broadly abundant terpenes.
Introduction
Diverse rearrangement, elongation and cyclization reactions are responsible for the divergent synthesis of natural terpenes and terpenoids.1-3 The resulting extraordinary complexity and diversity of possible skeletal structures makes terpenes and terpenoids the most diverse group of natural products.4, 5 Still, the steering of intermediary carbocations that determine the structural fate of these carbon skeletons remains up to this date an ongoing challenge in chemocatalysis. This applies in particular to challenging carbocation rearrangement reactions with multiple competing reaction pathways, such as terpene cyclization and isomerization.6, 7
The acidic isomerization of pinene stands as a representative of such reactions and is catalyzed with low selectivity by a variety of different acidic catalysts such as common Brønsted and Lewis acids, acid clays and zeolites, producing a wide mixture of different monoterpenoids (Figure 1A).8-10 Despite extensive research aimed at upgrading pinene to more valuable compounds, there has been limited success in selectively directing the carbocation pathways to yield singular products (Supporting Table S1). One of the most frequently targeted reactions is the conversion of α-pinene 1 or β-pinene 2 to camphene 3.9, 11 Here, the strained bicycle is preserved and the camphenyl cation 4 is formed from the initial pinanyl cation 5 by two concerted Wagner–Meerwein shifts, avoiding the secondary isobornyl cation 6 in the process (Figure 1B).12, 14 To achieve precise control over the selective termination of this secondary carbocation, it is essential to interrupt the concerted double 1,2 alkyl shift and inhibit divergent rearrangement pathways. Starting from the pinanyl cation 5, such pathways include the opening of strained bicycles to yield either the monocyclic menthenyl cation 7 or terpinene-4-yl cation 8, as well as the alkyl shift leading to the fenchyl carbocation 9. Steering such complex rearrangement networks necessitates strong support from the surrounding catalytic environment.13, 15, 16
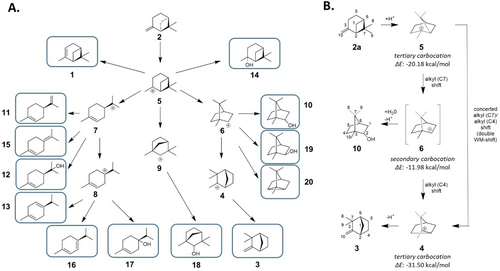
Proposed rearrangement pathways of (+)-(β)-pinene 2 a for the isomerization by engineering promiscuous squalene hopene cyclases. A) Acidic isomerization of β-pinene 2 is initiated by the protonation of the exocyclic alkene to yield the pinanyl cation 5, which can rearrange via various carbocationic intermediates (4–9) to a diverse set of valuable terpenes (see Supporting Figure S1 for details). B) The secondary isobornyl carbocation 6 does not represent a minimum on the potential energy surface during the isomerization of 2 a to 3, as indicated by the relative free energy of the carbocationic intermediates in the gas phase. Rather than settling at this highly reactive intermediate, the reaction undergoes a concerted double Wagner–Meerwein shift from the pinanyl cation 5 to the camphenyl cation 4.12, 13
Nature evolved enzymes that guide such reactive intermediates by precise arranged amino acids in their transition-state shape-complementary active sites.17 Terpene synthases, in particular, are believed to employ the concept of negative catalysis by effectively obstructing rearrangement along more favourable reaction pathways, thereby preventing the bifurcation of the reaction trajectory.18, 19 They can be differentiated by their initiation mechanism:3 The carbocation is either formed by abstraction of a diphosphate leaving group (class I) or by protonation of an alkene moiety via Brønsted acid catalysis (class II).20 While nature widely applies class I cyclases for the synthesis of monoterpenes, the application of substrates depending on diphosphate as a leaving group in catalysis is particularly challenging, due to fast hydrolysis under ambient conditions.21, 22
It has been extensively demonstrated that the highly acidic catalytic aspartic acid of the class II triterpene synthase squalene hopene cyclase (SHC) from Alicyclobacillus acidocaldarius (AacSHC)23 initiates carbocation-based rearrangements and cyclizations across varied terpene substrates.24-28 Exhibiting such promising properties, we set out to exploit the AacSHC as a biocatalyst for the selective isomerization of monoterpenes by steering challenging carbocation rearrangement reactions.
In this study we report our engineering of the AacSHC towards the enzymatic isomerization of β-pinene 2. First, we conducted semi-rational mutagenesis of active site residues, targeting hydrophobic and aromatic amino acids, in order to identify key positions for guiding cationic rearrangement pathways.27 Secondly, directed evolution through iterative site saturation mutagenesis generated a biocatalyst that allows further chaperoning of the desired rearrangement pathway via the reorganization of the aromatic residues of the monoterpene binding pocket, transforming the promiscuous SHC into a catalyst for highly selective (+)-borneol 10 a formation. Catalyzing this reaction in a highly chemo- and diastereoselective way demonstrates, that AacSHC can even facilitate the selective termination of the secondary isobornyl carbocation 6.
Results and Discussion
Switching Rearrangement Pathways by Single-Point Mutations
As a starting point of our efforts to accomplish the selective rearrangement of monoterpenes, we chose the enzymatic isomerization of β-pinene 2 by AacSHC. Pinene is found as a component of most essential oils and its availability as a strained bicyclic monoterpene makes it ideal as a starting substrate for our initial isomerization studies.29, 30 First conversions of the isomers (+)- and (−)-α-pinene 1 a (+)/1 b (−), as well as (+)- and (−)-β-pinene 2 a (+)/2 b (−) by the AacSHC wild-type showed the formation of the monoterpenes α-pinene 1, β-pinene 2, limonene 11, α-terpineol 12, γ-terpinene 13, and camphene 3 as main products. Depending on the substrate selected, the product distribution varied in part strongly (Supporting Figure S4). Due to its highest initial activity, substrate 2 a was chosen for further investigations. Furthermore, many additional products such as pinene hydrate 14, α-terpinolene 15, α-terpinene 16, terpinen-4-ol 17, fenchol 18, borneol 10, isoborneol 19, and tricyclene 20 were observed in trace amounts. This is in agreement with the acidic isomerization of β-pinene 2 by classical catalysts such as dowex® homogeneous acid catalyst, which lead to similar product mixtures of multiple monoterpenes (Supporting Figure S5).8-10
Cyclization reactions catalyzed by AacSHC are initiated through the protonation of the exposed alkene moiety, facilitated by an aspartic acid within the conserved DxDD motif. While mutating a single residue from this motif often results in a loss of catalytic function, it was intriguing to find that only by mutating all three aspartic acids could we entirely disrupt the activity for substrate 2 a (Supporting Figure S6). This triple mutation, termed AxAA (D374A/D376A/D377A), displayed no residual catalytic activity and served as a negative control in subsequent experiments (Further details in Supplementary Text 1).
To demonstrate the potential of AacSHC in directing this carbocationic rearrangement reaction, we targeted the selective conversion of 2 a into its individual main products. The active site of AacSHC is covered with mostly hydrophobic and aromatic residues that are responsible for the sophisticated prefolding of the natural substrate squalene and the stabilization of the intermediary carbocations of the polycylization reaction to hopene and hopanol (Supporting Figure S3).31 We targeted a set of active site amino acid positions (including L36, W169, I261, W312, F365, A419, Y420, V448, W489, G600, F601, F605, L607 and Y609) by introducing amino acids with similar hydrophobic properties in the hope of varying the orientation of the substrate without disturbing the hydrophobic composition of the active pocket. This initial screening resulted in the identification of the variants L36A, W312A and Y609W, responsible for shifting the selectivity of the main product to 53 % 11, 50 % 12 and 88 % 1 a, respectively (Figure 2). Furthermore, investigation of the second shell amino acids around those active site positions via saturation mutagenesis additionally resulted in the identification of the variant D442N with a selectivity for product 3 of 52 % (Supporting Figure S12). The discovery of those selectivity hotspots demonstrates that AacSHC can alter the rearrangement products only relying on single point mutations (Figure 2). The selectivity achieved in this process rivals or even exceeds those of known heterogeneous catalysts, highlighting the potential of AacSHC as a potent catalyst for monoterpene rearrangement reactions (Supporting Table S1). In addition to selectivity shifts, mutations such as the Y609W point mutation led to a 2.8-fold increase in activity.
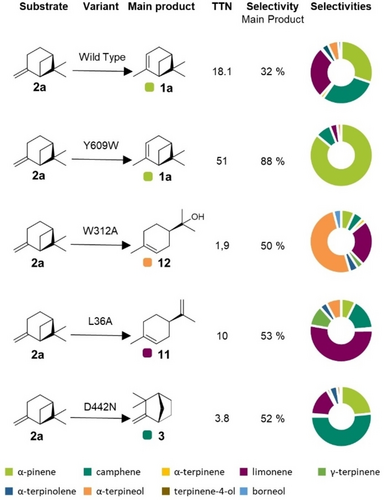
AacSHC variants for the selective carbocation rearrangement of (+)-β-pinene 2 a. All enzymes were characterized by the conversion of 2 mM 2 a using 10 mg mL−1 purified enzyme at pH 6 and 30 °C with 12 mM citric acid and 0.2 % (w/v) CHAPS and are compared by their total turnover number (TTN) after 40 h and their selectivity for the specified main product. The corresponding product distributions were quantified using external standards and are depicted in pie charts; further details can be found in Supporting Table S2 and Supplementary Chromatogram 5.
Saturation at positions L36, W312, D442, and Y609 yielded mostly active variants, but none surpassed the selectivity of the initially identified mutations (Supporting Figure S7–S11). No discernible correlation between amino acid properties and product selectivity was observed for the L36 and D442 saturations (Supporting Figure S8, S11). At position Y609, hydrophobic or aromatic amino acids like phenylalanine or valine increased formation of 1 a, with Y609W showing the highest activity and selectivity (Supporting Figure S7). Basic amino acids at Y609 and W312 led to a complete loss of activity, probably due to their proximity to the catalytic aspartic acid. Small hydrophobic amino acids like alanine or glycine favored the formation of product 12 when introduced at position W312, while larger hydrophobic or aromatic residues had heightened activity but low selectivity for product 12 (Supporting Figure S9). Most interestingly, changes induced by the saturation at position W312 also influenced the formation of the trace product borneol 10. This increase in selectivity for 10 follows the same trend as the formation of product 12 and peaks for variant W312A with 10.5 %, which represents an 8.7-fold increase in selectivity from the wild-type (WT) enzyme (Supporting Figure S10).
The secondary plant metabolite borneol 10, found in various essential oils, can be industrially obtained by the reduction of camphor, but this process lacks diastereoselectivity.32 Pure 10 possesses desirable properties like anti-inflammatory, pain relieving and anticoagulating effects, while its diastereomer isoborneol 19 exhibits toxic effects in higher concentrations.33, 34 Enzymatic synthesis of 10 from renewable pinene 1/2 offers a novel route for selective and straightforward one-step production of this valuable compound.
Single point mutations in the AacSHC demonstrate significant shifts in the product spectrum, highlighting its potential for promiscuous reactions. Similar phenomena have been observed in multiple enzymes, where single amino acid switches can completely alter catalytic properties.35-37 Furthermore, comparing our AacSHC variants with other biocatalysts like monoterpene synthases reveals that nature has evolved even more specific tools for monoterpene synthesis. While many monoterpene synthases produce product mixtures, there are highly selective examples, such as (4S)-(−)-limonene synthase (96 % (−)-limonene 11 b) and (+)-bornyl diphosphate synthase (75 % (+)-borneol 10 a), known for remarkable product and enantioselectivity.38, 39 To reach such levels of selectivity, we planned to further restructure the AacSHC active pocket.
Iterative Saturation Mutagenesis To Control Carbocation Rearrangements
The selective formation of borneol poses a significant challenge in the context of natural bornyl diphosphate formation. This intricate process entails the cyclization of geranyl diphosphate (GPP) under the catalytic influence of BPPS and the selective stabilization of carbocation 6 by the diphosphate counteranion (as illustrated in Supporting Figure S2).14 To accomplish this reaction independent of leaving groups we aimed to restructure AacSHC′s active site, aiming to selectively produce 10 from the renewable substrate 2 a. This task is particularly challenging since it requires the enzyme to terminate the rearrangement reaction selectively at the stage of the secondary isobornyl cation 6, while thwarting its Wagner–Meerwein shift into the energetically favoured tertiary camphenyl carbocation 4, without the support of the diphosphate group that functions as a directing counter anion in the natural BPPS reaction.12
To overcome this task by fine-tuning the active site of AacSHC we applied iterative site saturation mutagenesis employing the 22c-trick.40, 41 A total of 37 positions were targeted. In addition to focusing on the positions identified during the initial substrate screening, we also conducted iterative saturation mutagenesis targeting the second shell positions surrounding these amino acids. Furthermore, we saturated four positions along the substrate tunnel, as they displayed activity-enhancing characteristics for other promiscuous reactions.42, 43 Given our anticipation of a complex interplay among amino acids to selectively terminate carbocation 6, we consistently revisited active pocket positions that showed promising trends during saturation mutagenesis, aiming to address potential epistatic effects. The specific positions and their assignments can be found in Supporting Figure S13.
After 10 rounds of mutagenesis the octuple variant V10 (A306W/W312G/G315I/F365W/Y420F/V448I/Y609M/Y612F) was generated exhibiting a remarkable increase in selectivity for product 10 a from 1.2 % to 90.2 %, increasing the overall formation of this product 186-fold (Figure 3A). All mutations introduced are located in the active site near the catalytic aspartic acid D376 (Supporting Figure S14). The achieved selectivity for the acidic isomerization of monoterpenes by the eight mutations challenges even the selectivity of comparable monoterpene synthases like BPPS (75 %), without the need for substrate activation by a diphosphate leaving group.39 An additional feature is the highly diastereoselective carbocation termination by the nucleophilic attack of water which favors (+)-borneol 10 a over the diastereomer (−)-isoborneol 19 b with a de of 99.6 %, while the enantioselectivity of the strained bicyclic ring is retained.
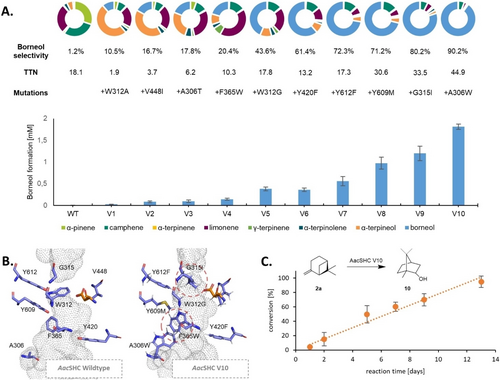
Directed evolution of AacSHC for the isomerization of (+)-β-pinene 2 a to (+)-borneol 10 a. A) Improvement of AacSHC in the rearrangement reaction to 10 by enzyme engineering. The AacSHC variants are compared based on their (+)-borneol 10 a formation within 40 h using 10 mg mL−1 purified enzyme. Error bars represent the standard deviation calculated from biological triplicates. The selectivities for the isomerization of 2 a are shown in the pie charts above. Further details can be found in Supporting Table S3 and Supplementary Chromatograms 6–17. B) Active pocket of AacSHC wild-type and variant 10 as predicted by Alphafold2 (catalytic aspartic acid shown as orange sticks, targeted residues shown as blue sticks). The results indicate a strong restructuring of the active pocket in correspondence with the modification of aromatic residues covering the active site at position A306, W312 and F365 (red circles; detailed view in Supporting Figure S14). C) Isomerization of 2 a to 10 a on a 1 L scale using 200 g L−1 enables the production of 1.3 g purified product with 99.6 % de.
Directed evolution often results in variants with complex interplay of the introduced mutations.44, 45 In this context we discovered a changing amino acid preference at positions A306 and W312 in the course of the directed evolution approach highlighting the epistatic effect of the introduced mutations.46 As an example, the mutation W312A initially represented the best starting mutation for the formation of 10 (Supporting Figure S10). However, in the process of further iterative mutagenesis, due to interactions with already introduced mutations, a change of the amino acid preference to glycine was observed, resulting in the doubling of the selectivity for 10.
We are aware that the application of Michaelis–Menten kinetics for the conversion of monoterpenes has to be seen critical due to possible limitations of substrate solubility, despite the use of cosolvents. However, kinetic evaluation indicates that not only the Km-value is nearly halved from 2.29 to 1.43 mM, the kcat also increases around 50 % from 1.37 to 2.12 h−1 implying an enhancement of the reaction environment beyond substrate affinity (Supporting Figure S17). Despite those improvements the catalytic efficiency remains on the lower end of the spectrum for enzyme catalyzed reactions. The low activity of terpene synthases (TTN in most cases below 103)47, 48 has been shown to be one of the major drawbacks of these biocatalysts in comparison to other enzyme families such as monooxygenases or transaminases (TTN of 105−7).49 In natural terpene biocatalysis synthetic control and precision is often preferred over the rate enhancement of the catalytic reaction.13 Furthermore, such limitations of enzyme activity are amplified for promiscuous reactions.50 To investigate the feasibility of the enzymatic pinene rearrangement on a preparative scale we converted 10 mM 2 a using 200 g/L resting E. coli cells expressing variant V10. After 14 days full conversion of the substrate was measured via GC and the product (+)-borneol 10 a with a yield of 84.3 % was purified using column chromatography, producing 1.3 g purified product and highlighting the potential of this engineered biocatalyst to perform reactions in gram-scale, due to its excellent stability enabling long term biotransformations, while exhibiting excellent diastereoselectivity of 99,6 % (Figure 3C).42, 51
In order to characterize the changes in the enzyme scaffold, we created a homology model of the V10 variant using the AlphaFold2 algorithm.52 The mutations introduced during directed evolution led to a major restructuring of the substrate binding pocket. This restructuring is mainly due to the enlargement of the active pocket near the catalytic aspartic acid by the mutation W312A, as well as the introduction of sterically demanding tryptophanes at position A306 and F365 (Figure 3B). Next we analyzed the shape of the substrate tunnels by means of CAVER analysis.53 The analysis indicated that the introduced mutations led to a narrowing of the substrate binding pocket, while the deeper region near the catalytic aspartic acid was widened further improving the binding of substrate 2 a (Supporting Figure S18).54 This reconstructed active pocket allows a new confinement for the promiscuous rearrangement of 2 a in a more controlled environment compared to the large cavity necessary for the space demanding prefolding of the natural substrate squalene.55
Reshaping of the Aromatic Active Site Residues Directs Monoterpene Rearrangement
By employing a deconvolution approach, which includes substrate dockings and the generation of all mutations in the form of single and double variants, we aimed to infer shifts in substrate positioning and gain insight into epistasis effects between the V10 mutations (Figure 4A, Supporting Table S4). With the exception of the W312A (9.3 %) and W312G (7.9 %) variants the single mutations from the directed evolution approach had little noteworthy effect on the formation of product 10. Position W312 is known as a key position for substrate binding of smaller molecules in close proximity to the catalytic D376 (4.3 Å)26 and appears to play a central role in the positioning of 2 a as well. The substrate coordination by W312 is also observable for the docking of 2 a in the active pocket of the AacSHC wild-type using YASARA (Supporting Figure S19).56 Despite the challenges of substrate docking and homology modeling in terpene cyclases,57 previous research has demonstrated the valuable insights that can be gained from such studies.47 Our results suggest that the tryptophan at position 312 plays a crucial role in coordinating 2 a, potentially stabilizing the carbocation at C3 and the intermediates 4 and 5 (Supporting Figure S19). This coordination could explain the formation of the major products 1 a and 3 in the WT reaction. Introducing smaller residues, such as alanine or glycine, at this position disrupts the original substrate binding, allowing for new coordination possibilities in the active pocket. As a result, variants W312A and W312G exhibit increased formation of the products 6 and 10.
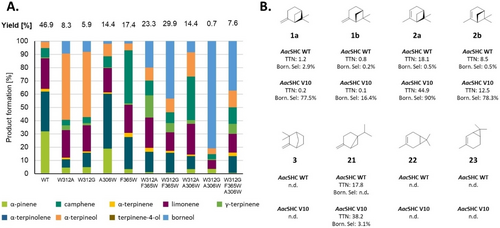
Deconvolution approach reveals the central role of aromatic active site residues for borneol 10 formation. A) Selected deconvolution variants of AacSHC V10. The variants are compared based on their product formation within 40 h using 0.2 mg mL−1 whole-cell catalyst. Conversions are depicted above the chart. For a complete list of all deconvolution variants see Supporting Table S4, as well as Supplementary Chromatograms 18–27. B) Conversion of different mono- and bicyclic monoterpenes by AacSHC wild-type and variant V10 reveal an increase in borneol selectivity, independent of the substrate used. All reactions were characterized by the conversion of 2 mM of the substrates 1 a/b, 2 a/b, 3, 11, 13, 15, 16, 21, 22 and 23 using 10 mg mL−1 of purified enzyme with 12 mM citric acid and 0.2 % (w/v) CHAPS for 40 h. The reactions are compared based on their total turnover number (TTN) and their product selectivity for product 10. Detailed product distributions of the complete substrate panel can be found in Supporting Figure S20, Supporting Table S5 and Supplementary Chromatogram 28.
To explore additional mutations contributing to the cooperative increase in (+)-borneol 10 a formation, we generated all possible double variants of the mutations during the iterative restructuring of the active pocket (Figure 4A, detailed information in Supporting Table S4). It can be seen that the introduction of the mutations W312A or W312G are prerequisite for enhanced 10 formation. Further cooperative enhancement was achieved by introducing new tryptophan residues at positions F365 or A306, with the variants W312G/F365W (43.2 %) and W312G/A306W (80.8 %) exhibiting the highest selectivity. However, the W312G/A306W variant displayed reduced catalytic activity. Thus, the W312G/F365W variant emerged as a preferable step towards generating the V10 variant. The mutations W312G, F365W, and A306W play a major role in determining selectivity for 10 by reshaping the pocket (Figure 3B) and repositioning substrate 2 a. Additional mutations identified during directed evolution (V448I, Y420F, Y612F, Y609M, G315I) seem to further enhance this effect or improve overall activity.
Subsequently, we investigated the applicability of the AacSHC V10 variant‘s ability to stabilize the secondary isobornyl cation 6 with other monoterpene substrates. We screened a panel of mono- and bicyclic monoterpenes, observing the rearrangement process induced by the restructured active site (Figure 4B, Supporting Figure S20). While the WT enzyme and variant V10 did not convert certain monocyclic and bicyclic monoterpenes, they successfully converted the pinene isomers 1 a/b and 2 a/b, as well as the bicyclic monoterpene sabinene 21. The V10 variant exhibited reduced conversion of substrates 1 a and 1 b due to active site adaptation for substrate 2 a. Directed evolution targeting a specific substrate often reduces substrate promiscuity.58 Interestingly, all reactions exhibited a significant shift towards increased formation of product 10, with fold increases ranging from 27 to 180. This pattern was not limited to pinene. The V10 variant facilitated the production of 3.1 % 10 from substrate 21, a result not seen with the wild-type enzyme. This outcome is notable, especially considering that the rearrangement process involves the opening of the strained bicycle and subsequent cyclization of the intermediary menthenyl cation 7 (Supporting Figure S21) (Further details in Supplementary Text 2).
In summary, our findings demonstrate that the active pocket of variant V10 not only stabilizes the secondary isobornyl carbocation 6, but also provides the necessary confinement to retain the bicyclic structure. The reorganization of aromatic active site residues improves substrate confinement and supports carbocation formation at carbon C3 via cation-π interactions, enabling highly selective termination of the secondary cation 6. Previous computational investigations by Tantillo have shown that the secondary isobornyl carbocation can be considered more like a transition states rather than an intermediate with energy minima.15 Achieving the formation of 10 from the secondary cation 6 requires a strong directing force for the correct rearrangement pathway, similar to the natural cyclization of (GPP) to bornyl diphosphate catalyzed by class I BPPS. The BPPS utilizes a complex mechanism involving allylic cation stabilization, counterion diphosphate binding, cation-π stabilization, and substrate pre-folding mediated by coordinated water. In contrast, our engineered catalyst generates high selectivity, surpassing that of common monoterpene synthases by relying on the rearrangement of the enzyme‘s aromatic cage structure, highlighting the remarkable potential of the AacSHC active pocket for carbocation stabilization and precise control through iterative enzyme engineering.
Computational Exploration of WT and V10 Variants by Means of Molecular Dynamics Simulations
To further unravel the role of the introduced mutations in the V10 variant towards the conversion of (+)-β-pinene 2 a into borneol 10, Molecular Dynamics (MD) simulations were performed on both WT and V10 variants (5 replicas of 500 ns for each system were performed, check Supporting Information for more details). A conformational population analysis in the absence of any ligand as shown in Figure 5 was performed to elucidate the conformational changes on the active site induced by the introduced mutations, as well as to correlate them with the selectivity towards 10 observed experimentally (Principal Component Analysis, PCA in Figure 5). In the case of AacSHC WT mainly 3 conformations of the active site residues are observed. Conformation A perfectly overlaps with the X-ray structure, thus showing the same orientation of the residues composing the active site. Conformation B displays a rotation and a displacement of F365 and W489, which provide a more compact active site with a close interaction of W489 with the catalytic D376. Finally, conformation C is similar to the X-ray structure but with the difference that the loop containing P444 adopts a more open conformation and exposes the active site directly to the bulk. In contrast, V10 variant is much less flexible as it only presents one main conformation similar to the X-ray structure (see conformation D in Figure 5). However, the introduced mutations reshape the active site pocket and presumably create a more suitable environment for the stabilization of the isobornyl carbocation 6.
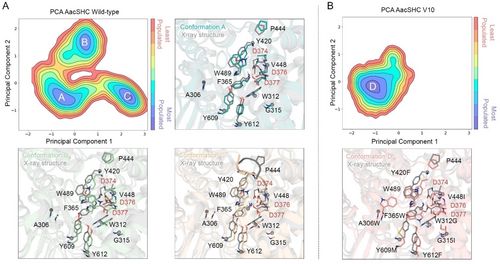
A) Conformational population analysis for AacSHC WT and overlay of the different conformations (i.e., conformation A, B, and C) with the X-ray structure (shown in gray, PDB code: 1UMP). In each overlay, the most relevant residues in terms of conformational differences are displayed, and the catalytic aspartic triad is labeled in red. B) Conformational population analysis for AacSHC V10 and overlay of the unique displayed conformation D with the X-ray crystal structure (shown in gray, PDB code: 1UMP). In the overlay, the catalytic aspartic triad is labeled in red, whereas the introduced mutations are highlighted with bold font.
Considering that the formation of borneol requires the isomerization of (+)-β-pinene 2 a and the subsequent hydration of the resulting isobornyl carbocation 6, we hypothesized that the introduced mutations in V10 could favor the confinement of water molecules within crucial regions of the active site cavity to facilitate the reaction. Mechanistic elucidation of BPPS activity has revealed comparable observations, where a firmly anchored water is situated within the active pocket of the native enzyme, forming an integral component of the essential active site contour necessary for template function.14 To elucidate this further, we first studied by means of independent Quantum Mechanical (QM) calculations in gas phase how the relative stability for the isomerization changes in the presence or absence of explicit water molecules (see Figure 6A, 6B and Supporting Information for more details). We considered the pinanyl carbocation 5 as starting point and performed QM-metadynamics to explore the isomerization of 5 towards 6 and 7 in the presence and absence of an explicit water molecule. In Figure 6A, the free energy surfaces of the isomerization process without and with an explicit water molecule are depicted. From these QM-metadynamics simulations, we observed that in the absence of water isobornyl carbocation 6 displays a rather high relative energy (ca. 3 kcal/mol higher than 5), while menthenyl 7 and camphyl 4 cation have much lower energy with respect to the initial carbocation, i.e., 13 and 21 kcal/mol, respectively (Figure 6A, and Supporting Figure S22). By adding a water molecule and restraining it at a minimum distance of 1.9 Å from the carbocation to avoid the hydration, the landscape drastically changes as the stability of 6 is substantially improved, whereas the barrier is similar in both cases (Figure 6A–B).
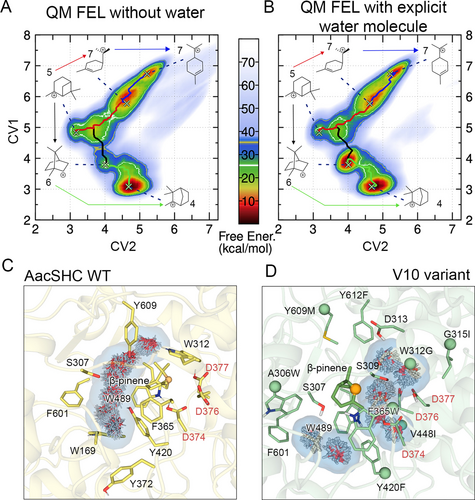
QM-metadynamics free energy surface (FES) of the isomerization process from pinanyl carbocation 5 to either menthenyl 7, or isobornyl 6, which can further evolve to camphyl 4 in the absence (A) or presence (B) of an explicit water molecule. The lowest-energy pathways are marked on top of the FES using different colors: red for the isomerization of 5 to 7, black for 5 to 6 and green for 6 to 4. Water cluster analysis in the active site pocket based on the most populated conformations of the substrate bound classical MD simulations for the C) WT and D) V10 variant. The most relevant residues for water stabilization are depicted and the carbon atom that will hold the positive charge after the protonation and isomerization of β-pinene 2 is depicted as an orange sphere. The catalytic aspartic triad is labeled in red, and green spheres represent the mutated residues.
The presence of the water molecule lowers the relative stability of isobornyl down to 4 kcal/mol (i.e., ca. 20 kcal/mol lower than 5, see Figure 6B), thus demonstrating the important role of water in the production of borneol 10.
As explained before, the introduced mutations in V10 reduce the flexibility of the active site pocket, but more importantly introduce more polar and aromatic residues that might contribute to stabilize the isobornyl carbocation 6. The QM-metadynamics calculations indicate that the presence of a water molecule specifically oriented close to the carbocation has a big impact on the relative stability. We therefore aimed to study how water is arranged in the active site cavity of both AacSHC WT and V10 variants when the substrate is bound in the active site. We run 5 MD replicas of 100 ns with (+)-β-pinene 2 a in the active site, applying harmonic constraints in the catalytic distance between 2 a and the catalytic aspartic acid D376 to keep the substrate close to the catalytic residues (Figure 6C). We reconstructed the binding Free Energy Landscape, considering the orientation of the substrate with respect to the catalytic aspartic triad (see computational methods in Supporting for more information, and Supporting Figure S23). We analyzed the water arrangement using the Solvent Structure and Thermodynamic Mapping (SSTMap) software59 on the most populated conformations on the FES (see Figure 6C–D). For WT, we observed that the residues surrounding the carbocation favor the presence of water molecules in the opposite face of the C2 carbon where the carbocation 6 will be generated after the isomerization (orange sphere in Figure 6C). In contrast, the mutations introduced in V10, that include aromatic residues around the catalytic triad, create the proper environment to position water molecules close to the carbon that will hold the positive charge after the isomerization of 5 into 6 (Figure 6D). More specifically, W312G in combination with F365W play a key role for water stabilization at the proper location for borneol production. Due to the extra space generated by W312G mutation, and more importantly the new positioning of the indole ring of the F365W mutation, the positioning of some water clusters close to the catalytic aspartic triad seems to be favored, as shown in Figure 6D. It is well-known that carbocations can be stabilized by negatively charged residues and/or by the partial negative charge of the π electrons of aromatic residues.60 This new conformation of W365 observed in V10 variant stabilizes the carbocation through π-CH interactions close to the catalytic aspartic triad (See Figure 5B and 6D). In contrast, in WT the presence of W312 favors the formation of a carbocation-π interaction with the substrate (see Figure 6C), thus hampering the presence of water molecules for the formation of the hydroxylation product (i.e. borneol 10). Surprisingly, water positioning of V10 appears to promote the arrangement of water molecules towards the C2 carbon‘s endo-direction. This suggests a possible link to the remarkable diastereoselectivity observed in the termination of carbocation 6. To gain a more in-depth understanding of this relationship, it is essential to analyze other variants, including the intermediate stages of iterative saturation mutagens. Remarkably, despite substantial restructuring of the active pocket, diastereoselectivity consistently aligns with the value observed in the final variant V10.
Overall, our combined QM-metadynamics and classical MD analysis underline the importance of the interaction between aromatic residues and explicit water molecules for the production of borneol 10 by lowering the energy of the isobornyl cation 6. Moreover, water analysis in substrate bound MD simulations revealed how the WT enzyme and V10 variants stabilize water molecules around the substrate, displaying how V10 variant creates a more suitable environment for the reaction to occur, mirroring the function of firmly anchored water molecules in enzymatic template of BPPS.
Conclusion
This study demonstrates directable control over cationic skeletal rearrangements by applying the remarkable potential of class II triterpene cyclases, particularly AacSHC, for precise carbocation stabilization. We have proven that this enzymatic catalyst can be engineered for target-oriented rearrangement of broadly abundant and renewable terpenes to valuable products through minor modification of active site residues.
Overall, our mechanistic investigations underline the importance of the interaction between aromatic residues and explicit water molecules for the production of borneol 10 by lowering the energy of the isobornyl cation 6. Furthermore, our analysis of water behaviour in substrate-bound MD simulations reveals how both the WT enzyme and V10 variants adeptly stabilize water molecules around the substrate. This insight elucidates the constructive role of water in guiding the cationic pathway, all while avoiding undesirable quenching of intermediary carbocations. In this context, V10 variant mirrors the function of the anchored water molecule in BPPS, stabilizing a water molecule that lowers the energy of the isobornyl carbocation 6, modifying the steering of the carbocationic rearrangement pathway.
This capability facilitates the streamlined synthesis of natural (+)-borneol 10 a from pinene on a gram scale, surpassing the selectivity achieved by all previously reported artificial and natural catalysts and highlighting the potential of the enzyme for the selective control of carbocations generated from unactivated substrates. By showcasing the ability of AacSHC to guide carbocation rearrangements in small monoterpenes, we aim to offer a versatile biocatalytic tool for synthesizing desired compounds, free from leaving-group constraints.
Acknowledgments
We thank K. Schell, B. M. Nestl, B. Aberle, L. Bengel and J. Wissner for fruitful discussions. We thank the Deutsche Forschungsgemeinschaft (DFG HA 1251/6-1) for research funding. B. Hauer, S. C. Hammer and J. Ludwig designed the overall research project. S. Diether and N. Kress performed preliminary experiments. J. Ludwig performed docking studies, conducted enzyme engineering and substrate scope studies. A. Schneider performed chemical synthesis. C. Curado, S. Ruiz-Barragán and S. Osuna performed MD/MQ-Simulations, PCA and free energy calculations. We thank the Generalitat de Catalunya for the consolidated group TCBioSys (SGR 2021 00487) and grant projects PID2021-129034NB-I00 and PDC2022-133950-I00 funded by Spanish MICIN. S. O. is grateful to the funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (ERC-2015-StG-679001, ERC-2022-POC-101112805 and ERC-2022-CoG-101088032), and the Human Frontier Science Program (HFSP) for project grant RGP0054/2020. C. Curado and S. Ruiz-Barragán were supported by a research grant from ERC-StG (ERC-2015-StG-679001) and HFSP grant (RGP0054/2020), respectively. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.