An Enzyme-Cleavable Solubilizing-Tag Facilitates the Chemical Synthesis of Mirror-Image Proteins
Yupeng Zheng
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorBaochang Zhang
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorWei-Wei Shi
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorXiangyu Deng
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorTong-Yue Wang
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDongyang Han
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYuxiang Ren
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorZiyi Yang
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYong-Kang Zhou
Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230027 China
Search for more papers by this authorJian Kuang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorZhi-Wen Wang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorProf. Shan Tang
Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230027 China
Search for more papers by this authorCorresponding Author
Prof. Ji-Shen Zheng
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Search for more papers by this authorYupeng Zheng
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorBaochang Zhang
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorWei-Wei Shi
Department of Chemistry, Tsinghua University, Beijing, 100084 China
These authors contributed equally to this work.
Search for more papers by this authorXiangyu Deng
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorTong-Yue Wang
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorDongyang Han
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYuxiang Ren
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorZiyi Yang
Department of Chemistry, Tsinghua University, Beijing, 100084 China
Search for more papers by this authorYong-Kang Zhou
Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230027 China
Search for more papers by this authorJian Kuang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorZhi-Wen Wang
Department of Chemistry, University of Science and Technology of China, Hefei, Anhui, 230026 China
Search for more papers by this authorProf. Shan Tang
Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230027 China
Search for more papers by this authorCorresponding Author
Prof. Ji-Shen Zheng
Department of Hematology, The First Affiliated Hospital of University of Science and Technology of China (USTC), MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, and Division of Life Sciences and Medicine, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230001 China
Search for more papers by this authorGraphical Abstract
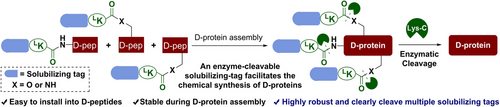
An enzyme-cleavable solubilizing tag was described for the chemical synthesis of mirror-image proteins. Solubilizing tags were easily installed on the side chain groups of DLys/DSer/DThr and the N-terminal α-amino group of D-peptides via an L-Lys linker. Solubilizing tags were impervious to various commonly used reagents in D-protein synthesis. After protein assembly, multiple solubilizing tags can be removed under denaturing conditions.
Abstract
Mirror-image proteins (D-proteins) are useful in biomedical research for purposes such as mirror-image screening for D-peptide drug discovery, but the chemical synthesis of many D-proteins is often low yielding due to the poor solubility or aggregation of their constituent peptide segments. Here, we report a Lys-C protease-cleavable solubilizing tag and its use to synthesize difficult-to-obtain D-proteins. Our tag is easily installed onto multiple amino acids such as DLys, DSer, DThr, and/or the N-terminal amino acid of hydrophobic D-peptides, is impervious to various reaction conditions, such as peptide synthesis, ligation, desulfurization, and transition metal-mediated deprotection, and yet can be completely removed by Lys-C protease under denaturing conditions to give the desired D-protein. The efficacy and practicality of the new method were exemplified in the synthesis of two challenging D-proteins: D-enantiomers of programmed cell death protein 1 IgV domain and SARS-CoV-2 envelope protein, in high yield. This work demonstrates that the enzymatic cleavage of solubilizing tags under denaturing conditions is feasible, thus paving the way for the production of more D-proteins.
Conflict of interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202318897-sup-0001-misc_information.pdf3.5 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Zhao, W. Lu, Curr. Opin. Chem. Biol. 2014, 22, 56–61;
- 1bM. Muttenthaler, G. F. King, D. J. Adams, P. F. Alewood, Nat. Rev. Drug Discov. 2021, 20, 309–325.
- 2
- 2aT. N. M. Schumacher, L. M. Mayr, D. L. Minor, M. A. Milhollen, M. W. Burgess, P. S. Kim, Science 1996, 271, 1854–1857;
- 2bM. Liu, M. Pazgier, C. Li, W. Yuan, C. Li, W. Lu, Angew. Chem. Int. Ed. 2010, 49, 3649–3652;
- 2cA. M. Levinson, J. H. McGee, A. G. Roberts, G. S. Creech, T. Wang, M. T. Peterson, R. C. Hendrickson, G. L. Verdine, S. J. Danishefsky, J. Am. Chem. Soc. 2017, 139, 7632–7639.
- 3H. N. Chang, B. Y. Liu, Y. K. Qi, Y. Zhou, Y. P. Chen, K. M. Pan, W. W. Li, X. M. Zhou, W. W. Ma, C. Y. Fu, Y. M. Qi, L. Liu, Y. F. Gao, Angew. Chem. Int. Ed. 2015, 54, 11760–11764.
- 4X. Zhou, C. Zuo, W. Li, W. Shi, X. Zhou, H. Wang, S. Chen, J. Du, G. Chen, W. Zhai, W. Zhao, Y. Wu, Y. Qi, L. Liu, Y. Gao, Angew. Chem. Int. Ed. 2020, 59, 15114–15118.
- 5
- 5aM. Paradís-Bas, J. Tulla-Puche, F. Albericio, Chem. Soc. Rev. 2016, 45, 631–654;
- 5bA. C. Conibear, E. E. Watson, R. J. Payne, C. F. W. Becker, Chem. Soc. Rev. 2018, 47, 9046–9068;
- 5cK. Harrison, A. S. Mackay, L. Kambanis, J. W. C. Maxwell, R. J. Payne, Nat. Chem. Rev. 2023, 7, 383–404;
- 5dJ. S. Zheng, S. Tang, Y. K. Qi, Z. P. Wang, L. Liu, Nat. Protoc. 2013, 8, 2483–2495;
- 5eM. Pan, S. Gao, Y. Zheng, X. D. Tan, H. Lan, X. L. Tang, D. M. Sun, L. N. Lu, T. Wang, Q. Y. Zheng, Y. C. Huang, J. W. Wang, L. Liu, J. Am. Chem. Soc. 2016, 138, 7429–7435.
- 6
- 6aG. M. Fang, Y. M. Li, F. Shen, Y. C. Huang, J. B. Li, Y. Lin, H. K. Cui, L. Liu, Angew. Chem. Int. Ed. 2011, 50, 7645–7649;
- 6bZ. Wang, W. Xu, L. Liu, T. F. Zhu, Nat. Chem. 2016, 8, 698–704;
- 6cJ. Weidmann, M. Schnölzer, P. E. Dawson, J. D. Hoheisel, Cell Chem. Biol. 2019, 26, 645–651;
- 6dB. Zhang, Y. Zheng, G. Chu, X. Deng, T. Y. Wang, W. W. Shi, Y. Zhou, S. Tang, J. S. Zheng, L. Liu, Angew. Chem. Int. Ed. 2023, 62, e202306270.
- 7A. J. G. Callahan, S. Travaline, T. L. Lozano Salazar, L. Hanna, S. Lee, Y.-C. Li, K. Tokareva, O. S. Swiecicki, J.-M. Loas, A. Verdine, G. L. McGee, J. H. Pentelute, ChemRxiv. 2023, DOI: 10.26434/chemrxiv-2023-x86xp.
- 8
- 8aJ. B. Li, S. Tang, J. S. Zheng, C. L. Tian, L. Liu, Acc. Chem. Res. 2017, 50, 1143–1153;
- 8bV. Agouridas, O. El Mahdi, V. Diemer, M. Cargoët, J. M. Monbaliu, O. Melnyk, Chem. Rev. 2019, 119, 7328–7443;
- 8cY. Tan, H. Wu, T. Wei, X. Li, J. Am. Chem. Soc. 2020, 142, 20288–20298.
- 9
- 9aE. C. Johnson, E. Malito, Y. Shen, D. Rich, W. J. Tang, S. B. H. Kent, J. Am. Chem. Soc. 2007, 129, 11480–11490;
- 9bJ.-X. Wang, G.-M. Fang, Y. He, D.-L. Qu, M. Yu, Z.-Y. Hong, L. Liu, Angew. Chem. Int. Ed. 2015, 54, 2194–2198;
- 9cM. Paradís-Bas, J. Tulla-Puche, F. Albericio, Org. Lett. 2015, 17, 294–297.
- 10C. Bello, K. Farbiarz, J. F. Möller, C. F. W. Becker, T. Schwientek, Chem. Sci. 2014, 5, 1634–1641.
- 11
- 11aJ. S. Zheng, M. Yu, Y. K. Qi, S. Tang, F. Shen, Z. P. Wang, L. Xiao, L. Zhang, C. L. Tian, L. Liu, J. Am. Chem. Soc. 2014, 136, 3695–3704;
- 11bC. Bello, S. Wang, L. Meng, K. W. Moremen, C. F. W. Becker, Angew. Chem. Int. Ed. 2015, 54, 7711–7715;
- 11cJ. S. Zheng, Y. He, C. Zuo, X. Y. Cai, S. Tang, Z. A. Wang, L. H. Zhang, C. L. Tian, L. Liu, J. Am. Chem. Soc. 2016, 138, 3553–3561;
- 11dS. Tang, C. Zuo, D. L. Huang, X. Y. Cai, L. H. Zhang, C. L. Tian, J. S. Zheng, L. Liu, Nat. Protoc. 2017, 12, 2554–2569;
- 11eD. L. Huang, C. Montigny, Y. Zheng, V. Beswick, Y. Li, X. X. Cao, T. Barbot, C. Jaxel, J. Liang, M. Xue, C. L. Tian, N. Jamin, J. S. Zheng, Angew. Chem. Int. Ed. 2020, 59, 5178–5184;
- 11fH. Wu, T. Wei, W. L. Ngai, H. Zhou, X. Li, J. Am. Chem. Soc. 2022, 144, 14748–14757;
- 11gH. Wu, Y. Tan, W. L. Ngai, X. Li, Chem. Sci. 2023, 14, 1582–1589.
- 12
- 12aZ. Tan, S. Shang, S. J. Danishefsky, Proc. Natl. Acad. Sci. USA. 2011, 108, 4297–4302;
- 12bY. C. Huang, Y. M. Li, Y. Chen, M. Pan, Y. T. Li, L. Yu, Q. X. Guo, L. Liu, Angew. Chem. Int. Ed. 2013, 52, 4858–4862;
- 12cY. Asahina, S. Komiya, A. Ohagi, R. Fujimoto, H. Tamagaki, K. Nakagawa, T. Sato, S. Akira, T. Takao, A. Ishii, Y. Nakahara, H. Hojo, Angew. Chem. Int. Ed. 2015, 54, 8226–8230;
- 12dS. K. Maity, G. Mann, M. Jbara, S. Laps, G. Kamnesky, A. Brik, Org. Lett. 2016, 18, 3026–3029;
- 12eM. T. Jacobsen, M. E. Petersen, X. Ye, M. Galibert, G. H. Lorimer, V. Aucagne, M. S. Kay, J. Am. Chem. Soc. 2016, 138, 11775–11782;
- 12fS. Tsuda, M. Mochizuki, H. Ishiba, K. Yoshizawa-Kumagaye, H. Nishio, S. Oishi, T. Yoshiya, Angew. Chem. Int. Ed. 2018, 57, 2105–2109;
- 12gJ. Liu, T. Wei, Y. Tan, H. Liu, X. Li, Chem. Sci. 2022, 13, 1367–1374.
- 13
- 13aD.-L. Huang, Y. Li, J. Liang, L. Yu, M. Xue, X.-X. Cao, B. Xiao, C.-L. Tian, L. Liu, J.-S. Zheng, J. Am. Chem. Soc. 2020, 142, 8790–8799;
- 13bY. Li, L.-J. Wang, Y.-K. Zhou, J. Liang, B. Xiao, J.-S. Zheng, Chin. Chem. Lett. 2023, 109033.
- 14T. J. Harmand, V. R. Pattabiraman, J. W. Bode, Angew. Chem. Int. Ed. 2017, 56, 12639–12643.
- 15L. Kerul, M. Schrems, A. Schmid, R. Meli, C. F. W. Becker, C. Bello, Angew. Chem. Int. Ed. 2022, 61, e202206116.
- 16R. Raijmakers, P. Neerincx, S. Mohammed, A. J. R. Heck, Chem. Commun. 2010, 46, 8827–8829.
- 17P. Giansanti, L. Tsiatsiani, T. Y. Low, A. J. R. Heck, Nat. Protoc. 2016, 11, 993–1006.
- 18S. Wang, J. Tam, B. Wang, R. Merripield, Int. J. Pept. Protein Res. 1981, 18, 459–467.
- 19A. H. Sharpe, K. E. Pauken, Nat. Rev. Immunol. 2018, 18, 153–167.
- 20J. Tang, J. X. Yu, V. M. Hubbard-Lucey, S. T. Neftelinov, J. P. Hodge, Y. Lin, Nat. Rev. Drug Discov. 2018, 17, 854–855.
- 21
- 21aT. Durek, P. F. Alewood, Angew. Chem. Int. Ed. 2011, 50, 12042–12045;
- 21bN. Ollivier, J. Vicogne, A. Vallin, H. Drobecq, R. Desmet, O. El Mahdi, B. Leclercq, G. Goormachtigh, V. Fafeur, O. Melnyk, Angew. Chem. Int. Ed. 2012, 51, 209–213;
- 21cD. T. Flood, J. C. J. Hintzen, M. J. Bird, P. A. Cistrone, J. S. Chen, P. E. Dawson, Angew. Chem. Int. Ed. 2018, 57, 11634–11639.
- 22Q. Wan, S. J. Danishefsky, Angew. Chem. Int. Ed. 2007, 46, 9248–9252.
- 23S. K. Maity, M. Jbara, S. Laps, A. Brik, Angew. Chem. Int. Ed. 2016, 55, 8108–8112.
- 24R. Pascolutti, X. Sun, J. Kao, R. L. Maute, A. M. Ring, G. R. Bowman, A. C. Kruse, Structure 2016, 24, 1719–1728.
- 25V. S. Mandala, M. J. McKay, A. A. Shcherbakov, A. J. Dregni, A. Kolocouris, M. Hong, Nat. Struct. Mol. Biol. 2020, 27, 1202–1208.
- 26G.-M. Fang, J.-X. Wang, L. Liu, Angew. Chem. Int. Ed. 2012, 51, 10347–10350.
- 27
- 27aG. B. Vamisetti, G. Satish, P. Sulkshane, G. Mann, M. H. Glickman, A. Brik, J. Am. Chem. Soc. 2020, 142, 19558–19569;
- 27bS. A. Abboud, M. Amoura, J. B. Madinier, B. Renoux, S. Papot, V. Piller, V. Aucagne, Angew. Chem. Int. Ed. 2021, 60, 18612–18618;
- 27cY. M. Li, Y. T. Li, M. Pan, X. Q. Kong, Y. C. Huang, Z. Y. Hong, L. Liu, Angew. Chem. Int. Ed. 2014, 126, 2230–2234;
- 27dH. S. Ai, M. S. Sun, A. J. Liu, Z. X. Sun, T. T. Liu, L. Gao, L. J. Liang, Q. Qu, Z. C. Li, Z. H. Deng, Z. B. Tong, G. C. Chu, X. L. Tian, H. T. Deng, S. W. Zhao, J. B. Li, Z. Y. Lou, L. Liu, Nat. Chem. Biol. 2022, 18, 972–980.
- 28
- 28aW. W. Shi, C. Shi, T. Y. Wang, Y. L. Li, Y. K. Zhou, X. H. Zhang, D. Bierer, J. S. Zheng, L. Liu, J. Am. Chem. Soc. 2022, 144, 349–357;
- 28bC. Zuo, W. W. Shi, X. X. Chen, M. Glatz, B. Riedl, I. Flamme, E. Pook, J. W. Wang, G. M. Fang, D. Bierer, L. Liu, Sci. China Chem. 2019, 62, 1371–1378;
- 28cW. Shi, T. Wang, Z. Yang, Y. Ren, D. Han, Y. Zheng, X. Deng, S. Tang, J. S. Zheng, Angew. Chem. Int. Ed. 2024, 63, e202313640.