Iron-Catalyzed Asymmetric α-Alkylation of 2-Acylimidazoles via Dehydrogenative Radical Cross-Coupling with Alkanes
Nian Xu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Contribution: Conceptualization (lead), Data curation (lead), Investigation (lead), Methodology (lead), Writing - original draft (lead)
Search for more papers by this authorMaoping Pu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Contribution: Formal analysis (lead), Software (lead)
Search for more papers by this authorHan Yu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Conceptualization (supporting)
Search for more papers by this authorGaofei Yang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Validation (lead)
Search for more papers by this authorCorresponding Author
Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Conceptualization (supporting), Supervision (supporting), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorNian Xu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Contribution: Conceptualization (lead), Data curation (lead), Investigation (lead), Methodology (lead), Writing - original draft (lead)
Search for more papers by this authorMaoping Pu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
These authors contributed equally to this work.
Contribution: Formal analysis (lead), Software (lead)
Search for more papers by this authorHan Yu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Conceptualization (supporting)
Search for more papers by this authorGaofei Yang
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Validation (lead)
Search for more papers by this authorCorresponding Author
Xiaohua Liu
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Contribution: Conceptualization (supporting), Supervision (supporting), Writing - review & editing (lead)
Search for more papers by this authorCorresponding Author
Xiaoming Feng
Key Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064 China
Search for more papers by this authorGraphical Abstract
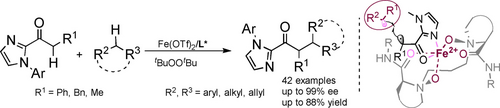
The asymmetric α-alkylation of acyclic carbonyls with hydrocarbons is of substantial interest and challenge. Herein, we achieved the enantioselective oxidative cross-coupling of 2-acylimidazoles with up to 99 % ee in moderate to good yields, thus providing an elegant access to optically active carbonyl compounds. Density functional theory calculations suggest a radical-radical cross-coupling pathway.
Abstract
The direct α-alkylation of acyclic carbonyls with nonactivated hydrocarbons through C(sp3)−H functionalization is both extremely promising and notably challenging, especially when attempting to achieve enantioselectivity using iron-based catalysts. We have identified a robust chiral iron complex for the oxidative cross-coupling of 2-acylimidazoles with benzylic and allylic hydrocarbons, as well as nonactivated alkanes. The readily available and tunable N,N′-dioxide catalysts of iron in connection with oxidants exhibit precise asymmetric induction (up to 99 % ee) with good compatibility in moderate to good yields (up to 88 % yield). This protocol provides an elegant and straightforward access to optically active acyclic carbonyl derivatives starting from simple alkanes without prefunctionalization. Density functional theory (DFT) calculations and control experiments were made to gain insight into the nature of C−C bond formation and the origin of enantioselectivity. We propose a radical-radical cross-coupling process enabled by the immediate interconversion between chiral ferric species and ferrous species.
Conflict of Interests
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202314256-sup-0001-misc_information.pdf30.2 MB | Supporting Information |
| anie202314256-sup-0001-xuj015.cif973.8 KB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aY. Liu, S.-J. Han, W.-B. Liu, B. M. Stoltz, Acc. Chem. Res. 2015, 48, 740–751;
- 1bT. B. Wright, P. A. Evans, Chem. Rev. 2021, 121, 9196–9242;
- 1cR. Cano, A. Zakarian, G. P. McGlacken, Angew. Chem. Int. Ed. 2017, 56, 9278–9290.
- 2
- 2aX. Tong, F. Schneck, G. C. Fu, J. Am. Chem. Soc. 2022, 144, 14856–14863;
- 2bD. Vargová, I. Némethová, K. Plevová, R. Šebesta ACS Catal. 2019, 9, 3104–3143.
- 3R. K. Zhang, K. Chen, X. Huang, L. Wohlschlager, H. Renata, F. H. Arnold, Nature 2018, 565, 67–72.
- 4For selected examples of Li's work in CDC reactions, see:
- 4aZ. Li, C.-J. Li, J. Am. Chem. Soc. 2005, 127, 3672–3673;
- 4bZ. Li, C.-J. Li, J. Am. Chem. Soc. 2006, 128, 56–57;
- 4cY. Zhang, C.-J. Li, J. Am. Chem. Soc. 2006, 128, 4242–4243;
- 4dY. Zhang, C.-J. Li, Eur. J. Org. Chem. 2007, 4654–4657;
- 4eZ. Li, C. Lin, C.-J. Li, Angew. Chem. Int. Ed. 2007, 46, 6505–6507.
- 5
- 5aW. Zhang, S. Yang, Z. Shen, Adv. Synth. Catal. 2016, 358, 2392–2397;
- 5bJ. Xie, Z. Huang, Angew. Chem. Int. Ed. 2010, 49, 10181–10185;
- 5cG. Zhang, Y. Zhang, R. Wang, Angew. Chem. Int. Ed. 2011, 50, 10429–10432;
- 5dG. Wang, X. Xin, Z. Wang, G. Lu, Y. Ma, L. Liu, Nat. Commun. 2019, 10, 559–567.
- 6
- 6aF. Benfatti, M. Capdevila, L. Zoli, E. Benedettoa, P. G. Cozzi, Chem. Commun. 2009, 5919–5921;
- 6bC. Guo, J. Song, S. Luo, L.-Z. Gong, Angew. Chem. Int. Ed. 2010, 49, 5558–5562;
- 6cY.-L. Zhao, Y. Wang, X.-Q. Hu, P.-F. Xu, Chem. Commun. 2013, 49, 7555–7557;
- 6dW. D. Cao, X. H. Liu, R. X. Peng, P. He, L. L. Lin, X. M. Feng, Chem. Commun. 2013, 49, 3470–3472;
- 6eX. Liu, C. Zhao, R. Zhu, L. Liu, Angew. Chem. Int. Ed. 2021, 60, 18499–18503.
- 7
- 7aP. Wang, H. Lin, Y. Zhai, Z. Han, L.-Z. Gong, Angew. Chem. Int. Ed. 2014, 53, 12218–12221;
- 7bL.-F. Fan, S.-W. Luo, S.-S. Chen, T.-C. Wang, P.-S. Wang, L.-Z. Gong, Angew. Chem. Int. Ed. 2019, 58, 16806–16810;
- 7cT.-C. Wang, P.-S. Wang, L.-Z. Gong, Sci. China Chem. 2020, 63, 454–459.
- 8L. Lv, Z. Li, Top. Curr. Chem. 2016, 374, 38–76.
- 9S. Narute, D. Pappo, Org. Lett. 2017, 19, 2917–2920.
- 10
- 10aM. A. Halcrow, Crystals 2016, 6, 58–77;
- 10bS. M. Brewer, K. R. Wilson, D. G. Jones, E. W. Reinheimer, S. J. Archibald, T. J. Prior, M. A. Ayala, A. L. Foster, T. J. Hubin, K. N. Green, Inorg. Chem. 2018, 57, 8890–8902.
- 11
- 11aK. M. Rosso, J. J. Morgan, Geochim. Cosmochim. Acta. 2002, 66, 4223–4233;
- 11bM. Chou, C. Creutz, N. Sutin, J. Am. Chem. Soc. 1977, 99, 5615–5623;
- 11cH. Zou, S.-F. Zhu, Progr. Chem. 2020, 32, 1766–1803.
- 12
- 12aT. Huang, X. H. Liu, J. W. Lang, J. Xu, L. L. Lin, X. M. Feng, ACS Catal. 2017, 7, 5654–5660;
- 12bH. K. Wang, Y. Xu, F. Q. Zhang, Y. B. Liu, X. M. Feng, Angew. Chem. Int. Ed. 2022, 61, e202115715;
- 13For selected examples of chiral N,N′-dioxides catalyzed reactions, see:
- 13aX. H. Liu, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2011, 44, 574–587;
- 13bX. H. Liu, L. L. Lin, X. M. Feng, Org. Chem. Front. 2014, 1, 298–302;
- 13cX. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin, X. M. Feng, Acc. Chem. Res. 2017, 50, 2621–2031;
- 13dX. H. Liu, S. X. Dong, L. L. Lin, X. M. Feng, Chin. J. Chem. 2018, 36, 791–797;
- 13eZ. Wang, X. H. Liu, X. M. Feng, Aldrichimica Acta 2020, 53, 3–10;
- 13fW. D. Cao, X. H. Liu, X. M. Feng, Chin. Sci. Bull. 2020, 65, 2942–2951;
- 13gM.-Y. Wang, W. Li, Chin. J. Chem. 2021, 39, 969–984;
- 13hD.-F. Chen, L.-Z. Gong, Org. Chem. Front. 2023, 10, 3676–3683;
- 13iS. X. Dong, X. H. Liu, X. M. Feng, Acc. Chem. Res. 2022, 55, 415–428;
- 13jS. X. Dong, W. D. Cao, M. P. Pu, X. H. Liu, X. M. Feng, CCS Chem. 2023, DOI: 10.31635/ccschem.023.202303417;
- 13kL. Z. Hou, X. H. Liu, W. D. Cao, X. M. Feng, ChemCatChem 2023, e202300893;
- 13lY.-M. He, Y.-Z. Cheng, Y. D. Duan, Y.-D. Zhang, Q.-H. Fan, S.-L. You, S. Z. Luo, S.-F. Zhu, X.-F. Fu, Q.-L. Zhou, CCS Chem. 2023, DOI: 10.31635/ccschem.023.202303347.
- 14
- 14aS. Hong, Y.-M. Lee, K.-B. Cho, K. Sundaravel, J. Cho, M. J. Kim, W. Shin, W. Nam, J. Am. Chem. Soc. 2011, 133, 11876–11879;
- 14bP. S. Steinlandt, L. Zhang, E. Meggers, Chem. Rev. 2023, 123, 4764–4794.
- 15K. J. Schwarz, C. Yang, J. W. B. Fyfe, T. N. Snaddon, Angew. Chem. Int. Ed. 2018, 57, 12102–12105.
- 16T. Tanaka, K. Hashiguchi, R. Yazaki, T. Ohshima, ACS Catal. 2018, 8, 8430–8440.
- 17For selected examples of Meggers's work using 2-acylimidazole in radical alkylation reactions, see:
- 17aH. Huo, X. Shen, C. Wang, L. Zhang, P. Rose, L.-A. Chen, K. Harms, M. Marsch, G. Hilt, E. Meggers, Nature 2014, 515, 100–103;
- 17bX. Huang, R. D. Webster, K. Harms, E. Meggers, J. Am. Chem. Soc. 2016, 138, 12636–12642;
- 17cH. Huo, C. Fu, K. Harms, E. Meggers, J. Am. Chem. Soc. 2014, 136, 2990–2993;
- 17dP. Xiong, M. Hemming, S. Ivlev, E. Meggers, J. Am. Chem. Soc. 2022, 144, 6964–6971;
- 17eN. Demirel, J. Qin, S. I. Ivlev, K. Harms, E. Meggers, Adv. Synth. Catal. 2021, 363, 4695–4700;
- 17fP. S. Steinlandt, W. Zuo, K. Harms, E. Meggers, Chem. Eur. J. 2019, 25, 15333–15340.
- 18For selected examples of work using 2-acylimidazole in radical alkylation reactions
- 18aK. Zhou, Y. Yu, Y.-M. Lin, Y. Li, L. Gong, Green Chem. 2020, 22, 4597–4603;
- 18bX. Huang, Q. Zhang, J. Lin, Nat. Catal. 2019, 2, 34–40;
- 18cQ. Zhang, X. Chang, L. Peng, C. Guo, Angew. Chem. Int. Ed. 2019, 58, 6999–7003.
- 19G. M. Fusi, S. Gazzola, U. Piarulli, Adv. Synth. Catal. 2022, 364, 696–714.
- 20Deposition number 2223628 (for 3 aa) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.