Neutral and Anionic Square Planar Palladium(0) Complexes Stabilized by a Silicon Z-Type Ligand
Graphical Abstract
The first neutral and anionic square planar (sp) Pd(0) (ate) complexes are synthesized and fully characterized. Such species, which have been proposed as intermediates in Pd-catalyzed cross coupling reactions, are stabilized using a Si(IV) based Lewis acidic ambiphilic Z-type ligand, which causes a strong Pd−Si Z-type interaction as confirmed by DFT derived methods.
Abstract
Anionic [Pd(0)−X]− ate complex were proposed as key intermediates in Pd-catalyzed cross-coupling for decades, but their isolation remained elusive. Herein, a chelating Lewis acidic bis(amidophenolato)silane is introduced as a strong Z-type ligand which enables the characterization of the first anionic [Pd(0)−X]− ate complex. Intriguingly, these compounds and the neutral L−Pd(0) analogs exhibit a square planar coordination that is highly unusual for a d10 metal. Theoretical methods scrutinize the interaction between the Lewis acidic Si(IV) center and the late transition metal, while reactivity studies shed light on the potential role of anionic additives in oxidative addition reactions.
The involvement of anionic Pd(0) intermediates in cross-coupling reactions like the Heck, Suzuki, Stille or Buchwald–Hartwig reactions is widely accepted in organometallic catalysis.1 While increased yields were often noticed upon the addition of halide salts, corresponding intermediates were never isolated, and the molecular explanation of the effect remained debated.2 In pioneering work, Amatore and Jutand used electrochemistry and 31P NMR spectroscopy to justify the rate acceleration in the oxidative addition by the formation of [Pd(0)−X]− ate complexes (Scheme 1a).3 Theoretical studies corroborated the involvement of such and various other anionic species in the catalytic cycle.4 As an alternative, Joy and Shaik suggested that the beneficial role of anionic additives could be attributed to oriented external electric fields (OEEFs) from anions that are not directly bound, but hold in proximity to the Pd(0) center by non-covalent interactions.5 Karaghiosoff and Koszinowski extended the experimental characterization by using electron-poor phosphines as co-ligands and could prove the existence of Pd(II) ate complexes by mass spectrometry and NMR analysis.6 In general, isolated examples of anionic group 10 M0 (M0=Ni, Pd, Pt) complexes are scarce in the literature (Scheme 1b), and the full characterization of a [Pd(0)−X]− ate complex remained elusive.7
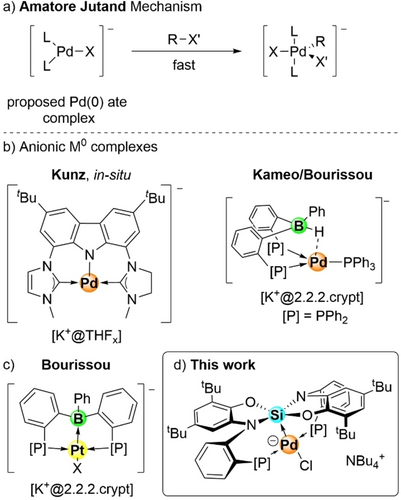
a) Pd(0) ate complexes as proposed as possible intermediates in the Amatore Jutand catalytic cycle of cross-coupling reactions. b) Examples of anionic d10 complexes. c) The first isolated anionic sq-Pt(0) ate-complex with a boron Z-type ligand. d) Isolated anionic sp-Pd(0) ate complex with a Si(IV) Z-type ligand presented in this work.
Ambiphilic ligands incorporating Lewis acidic sites to function as σ-accepting Z-type ligands have risen in popularity in the past 20 years.8 A substantial alteration of the reactivity at the metal center was achieved by adjusting the electron density at the transition metal through the Z-type interaction.9 Only recently, the Bourissou group used this concept to isolate the first square-planar (sp) anionic d10 Pt complex by employing a diphosphine-borane as an ambiphilic L2Z-ligand (Scheme 1c).7f While group 13 elements are well established as Z-type ligands due to the inherent Lewis acidic features caused by their empty pz-orbital, much less is reported in such context on using neutral group 14 based Lewis acids.10 In recent years, we developed several examples of Lewis superacidic neutral silanes with application potential in catalysis and in the stabilization of intermediates of interest in organic synthesis.11
Although common neutral silanes possess comparably low fluoride ion affinities (FIA, general Lewis acidity), they cause strong 31P NMR shifts of coordinated phosphine oxide in the Gutmann Beckett test, meaning they inherit a highly effective Lewis acidity.12 Hence, we were encouraged to probe the putatively strong effect of Si(IV) Lewis acids on d-block metals. Aiming for a strong interaction between the transition metal and the Lewis acid in Z-type, we here report the synthesis of an ambiphilic bisphosphine-Si(IV) ligand. The corresponding Pd(0) complex exhibits strong push-pull binding and enables the isolation of the first neutral and anionic sp Pd(0) complexes (Scheme 1d).
Our study was launched by designing a ligand that combines a Si(IV)-centered acceptor with P-donor functionalities. We were inspired by van der Vlugt's redox active diphenylphosphanyl aminophenol (PNO−H2) 1, which had been successfully employed as a ligand for hetero-bimetallic Ni−Au complexes (Figure 1).13 Reacting 1 with silicon tetrachloride cleanly furnished the protonated chloridosilicate [H][2-Cl] with a characteristic 29Si NMR shift (δ=−96.3 ppm), implying a penta-coordinated Si species. Its structure was confirmed by scXRD analysis (see ESI for details). Deprotonation with tetramethyl piperidine (TMP) and chloride abstraction with either TMSOTf or NaBArF20 cleanly afforded silane 2. Alternatively, the latter could be synthesized by reaction of 1 with tris(dimethylamino)silane to yield the dimethyl amine adduct [2-NHMe2], which, in comparison to [H][2-Cl], exhibits two signals in the 31P{1H} NMR spectrum instead of one. This is caused by hydrogen bonding of one phosphine arm to the coordinated amine, leading to a rigid structure, as confirmed in the solid state structure (see ESI for scXRD). Abstraction of the amine with the oligomeric Lewis superacid [Si(catCl)2]n cleanly furnished 2.
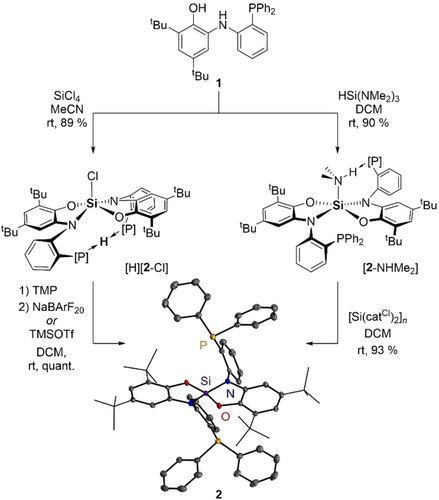
Synthesis of the Z-type ligand 2 from phosphanyl aminophenol 1 and ORTEP plot of the solid state structure of 2 (hydrogen atoms and solvent molecules omitted for clarity, vibrational ellipsoids displayed at 50 %).
No coordination of the phosphines to the Lewis acidic center can be observed in 2, resulting in a tetrahedral geometry at the silicon center, as confirmed by 29Si NMR spectroscopy (δ(29Si)=−44.2 ppm) and scXRD (Figure 1).14 Reacting 2 or [2-NHMe2] with cyclopentadienyl allyl palladium(II) resulted in the formation of a pristine compound 3 with a single resonance in the 31P NMR spectrum, indicating a symmetric coordination of Pd(0) that is formed by reductive elimination (Figure 2a). A first indication of a silicon-palladium interaction in 3 was observed by 29Si NMR spectroscopy, showing a high-field shifted signal (δ(29Si)=−83.6 ppm) as a triplet from 2J coupling (6.3 Hz) of the 29Si nucleus with the two equivalent phosphorus nuclei. Analysis of single-crystals by X-ray diffraction revealed a Si−Pd bond length of 2.3707(6) Å in the T-shaped complex (Figure 2b), which is significantly shorter than the Si−Pd bond lengths for previously observed dative Pd−Si bonds (Grobe et al.: 3.875 Å, Bourissou et al.: 2.9770(0) Å).15 The Si−Pd bond length is even comparable to the P−Pd bonds (2.2909(6) and 2.2939(6) Å), indicating equal contributions of Pd by σ-acceptor (P-donors) and σ-donor (Si-acceptor) interaction. The calculated ratio of bond-length and covalent radii (r=0.95) is within the range of a Z-type interaction.8b, 16
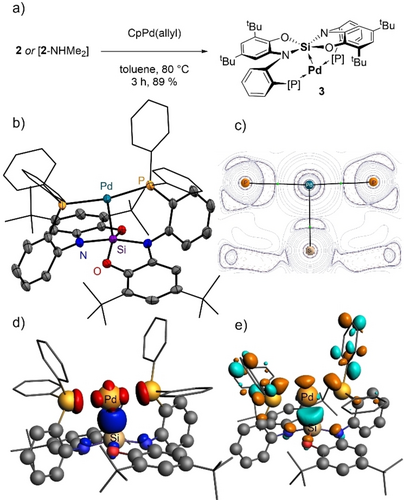
a) Synthesis of 3 through reductive elimination of CpPd(allyl) in the presence of silane 2. b) ORTEP plot of the solid-state structure of 3. Hydrogen atoms and disordered PPh2 units were omitted for clarity; vibrational ellipsoids are displayed at 50 %. c) View of the P2−Pd−Si interaction in complex 3 as obtained by QTAIM analysis, displaying the Laplacian of the electron density, bond paths and bond critical points. d) ETS-NOCV deformation density Δρ of the NOCV corresponding to the Pd−Si interaction (charge flow from red to blue, BP86-D3BJ/TZVP//PBEh-3c, 0.045 e/A3) e) LUMO of 3 (BP86-D3BJ/TZVP//PBEh-3c, 0.045 e/A3).
A series of theoretical analyses was employed to investigate the Pd−Si bond in 3. The Wiberg bond index of the Si−Pd bond is 0.43, which is even higher than the Pd−P WBIs (0.42). Natural charges obtained by NPA show a diminished positive charge at the Si center (2.038 vs. 2.354 in 2), underlining a Pd->Si charge flow. Second-order perturbation theory analysis manifests the Pd->Si donation from a filled d-orbital into a non-Lewis type Si-centered NBO (61 % s-; 39 % p-character) with a stabilization energy of 112 kcal/mol. QTAIM analysis of 3 revealed a bond path between Si and Pd. The electron density at the bond critical point (ρ(rBCP(Si−Pd)=0.074) is substantially larger as in the previously reported Z-type interaction of a less Lewis acidic silane with gold (ρ(rBCP(Si−Au)=0.021),17 but of similar magnitude to a strong Pt−Sb(V) interaction.18 The negative Laplacian of the electron density ∇2ρ(rBCP) and other topological descriptors attribute a pronounced covalent character (shared interaction) to the Si−Pd interaction (Figure 2c and section 2 f in the ESI). ETS-NOCV calculations (BP86-D3BJ/TZ2P//PBEh-3c) further explained the bond formation between the neutral Pd(0) and the ligand. The major contribution to the total overall orbital interaction energy is provided by the NOCV orbital with deformation density Δρ shown in Figure 2d (ΔEorb1=−65.5 kcal mol-1). It reflects the push-pull interaction of the ligand through the electron flow of the phosphines to the Pd center with simultaneous electron donation from filled dz2 and dx2-y2 orbitals at Pd in the pz-type acceptor and antibonding σ*(Si−N/O) orbital of the silane. We note in passing that the pseudo-four-fold symmetry of the acceptor unit might allow interactions of δ-symmetry (dx2-y2 orbital as donor), that would be symmetry-forbidden for the three-fold symmetric acceptor sites with the established group 13 based Z-type ligands (see Figure S9). While the results obtained by calculations strongly indicate the formation of a dative Pd->Si bond, we wanted to address the possibility that the potentially redox non-innocent amidophenolate could contribute electrons to the Si center, resulting in a formal Si(II)->Pd interaction in complex 3. While a bond-length contraction in the OCCN scaffold of 3 can in fact be observed in the solid state structures after coordination of Pd compared to silane 2, the calculated metric oxidation states (MOS) of −1.92 in the ligands indicate only minor contributions of such bonding mode (see ESI, section 3c). Furthermore, the computed triplet state geometries strongly diverged from the experimental molecular structures, while no broken symmetry DFT solutions could be found. The Pd−Si interaction can therefore be seen as a push-pull system, with electron withdrawing effects at the Si(IV) center strongly dominating any silylene donating effects. Expected trends in the reactivity of 3 were derived from the frontier molecular orbitals. While the HOMO is located at the ligand backbone (see ESI for details), the LUMO shows strong contributions at Pd, indicating electrophilicity at the formally electron rich d10 metal center (Figure 2e).
Indeed, while 3 behaves inert in reactions with electrophiles like aryl halides at room temperature, it readily undergoes reactions with nucleophiles in solution (Figure 3a). These findings are in line with other Z-type Pd complexes that do not undergo oxidative addition with aryl iodides at room temperature.19 We first investigated the reactivity towards the weakly σ-donating but π-accepting CO. Pressurizing a solution of 3 with 5 bar of CO results in an increase of the observed 2J coupling in the 29Si NMR spectrum (6.3 Hz ->13.4 Hz). A resonance at 185.2 ppm in the 13C{1H} NMR spectrum states the formation of [3-CO], in line with the DFT-computed free energy of this process (ΔG0=−24.1 kJ/mol).20 The binding equilibrium depends on the CO pressure and is reversed under reduced pressure. A weak back-donation of the Pd center to the CO ligand was confirmed by ETS-NOCV analysis (for more details, see ESI). While isolation of [3-CO] rendered impractical, the reaction of 3 with cyclohexyl isocyanide (CNCy) cleanly furnished complex [3-CNCy], which could be fully characterized. The 2JSi-P coupling in the 29Si NMR spectrum (δ(29Si)=−78.4 ppm) increased to 37.3 Hz, indicating a strong coordination of CNCy to 3. As already suggested for [3-CO], no significant π-backbonding takes place in [3-CNCy], as noted from the blue-shifted ν(CN) band in the IR spectrum (2178 cm−1 vs. 2136 cm−1 in free CNCy). Interestingly, this behavior contrasts a previously reported isocyanide Z-type Pd(0) complex, which showed a red-shifted IR band for the respective stretching modes due to remaining π-back binding.21 The molecular structure of [3-CNCy] in the solid state revealed the coordination of the Lewis basic isocyanide to occur at the Pd, as already supposed by the LUMO in 3. Remarkably, although being a d10 Pd(0) species, the coordination geometry at the Pd is square planar (sp) (Σ(Pd−X)°=360.05(7)°). To our knowledge, this structure represents the first report of a sp Pd(0) complex.
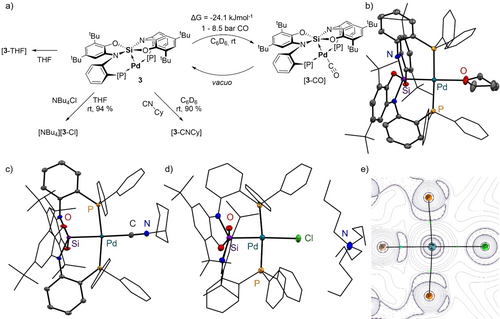
a) Reactivity of 3 with different nucleophiles. ORTEP plots of the solid state structure of b) [3-THF], c) [3-CNCy] and d) [NBu4][3-Cl] (hydrogen atoms, non-coordinating solvent molecules and disordered positions are omitted for clarity, vibrational ellipsoids displayed at 50 %). e) View of the P2−Pd−Si interaction in complex [3-Cl] as obtained by QTAIM analysis, displaying the Laplacian of the electron density, bond paths and bond critical points.
When dissolved in THF, strongly shifted resonances of 3 in the 1H NMR spectrum indicate coordination of the σ-donating solvent to the complex. These findings are supported by an increase in the observed 2JSi-P coupling (9.3 Hz) and were proven by scXRD analysis of the donor-adduct [3-THF] (Figure 3b). As for the π-accepting CNCy complexes, the coordination geometry at the Pd(0) center is sp (Σ(Pd−X)=360.13(5)). The weakly bound THF (computed ΔG0=−7.2 kJ/mol)22 is easily replaced with a chloride anion, resulting in the formation of the anionic Pd(0) complex [NBu4][3-Cl], which was isolated and fully characterized (Figure 3c). Again, the increased σ-donation of the Pd towards the Si center is reflected in an increased 2JSi-P coupling (27.3 Hz), as well as in a shortened Pd−Si bond (2.3185(14) Å, cf. 2.3707(6) Å in 3). As its neutral counterparts, the coordination environment at Pd is almost ideally sp (Σ(Pd−X)°=361.46(5)°). The compound [NBu4][3-Cl] thus not only represents a unique example of an isolated anionic Pd(0) ate complex but also features the unusual sp geometry. A relaxed surface scan along the Si−Pd−Cl angle underlines the energetically favored sp-coordination geometry (ESI section 2a). ETS-NOCV analysis illustrates the dominant σ-donation of the chloride fragment into the Pd−Si bond (see ESI for details). NPA of [3-Cl]− supports the d10 configuration of the Pd atom (0.0537 in [3-Cl]− vs. −0.1388 in 3), and a significant charge transfer to the Lewis acidic Si center (1.868 vs 2.038 in 3). In line with all experimental and spectroscopic findings, the WBI for the Si−Pd increased to 0.69 (cf. 0.43 for 3). An increased electron density at the bond critical point (ρ(rBCP(Si−Pd)=0.093) with an even stronger covalent character compared to 3 was found by the QTAIM analysis (Figure 3e and Section 2f in the ESI).
To probe the effect of coordination or charge states on the rates of oxidative addition with arylhalides, the reactivity of 3 (in C6D6), [3-(THF)] and [3-Cl]− towards PhX (X=Cl, Br, I) was compared. For all three complexes, no reaction with phenyl chloride or bromide took place, even at elevated temperatures (80 °C).
However, the oxidative addition of phenyl iodide took place at 60 °C, with a slightly increasing rate from 3 (in C6D6)<[3-(THF)]<[3-Cl]− (Table S1). Interestingly, scXRD analysis of the final product of oxidative addition revealed a phenyl group migrated to the Lewis acidic silicon center, yielding [Ph-2-Pd(II)-I] (see ESI, Figure S6 for details). Although a significant boost in reactivity for the anionic ate-complex, as would be expected according to the Amatore-Jutand cycle, was not observed, the trend generally agrees with the beneficial effect of directly bound nucleophile additives to the Pd-center, most pronounced for anions. Evidently, the particular type of ligand 2, primarily aiming for the stabilization of electron rich Pd centers, or potential cooperative and solvent effects must not be ignored while speculating on any generality of this statement.23
In conclusion, we successfully employed a Si(IV) based Z-type ligand with highly effective Lewis acidity for the complexation of Pd(0). A pronounced Pd−Si interaction, confirmed by spectroscopical and theoretical methods, causes significant changes in the reactivity of this late transition metal. The isolation and characterization of the first (anionic) sp Pd(0) complex is achieved, providing a missing piece of the Amatore/Jutand catalytic cycle in cross coupling reactions. A slightly increased rate of the anionic d10 complexes in the oxidative addition of phenyl iodide was observed, leaving room for speculation whether this trend is general, exclusive to this specific system, outbalanced from other effects, or instead promoted according to Shaik's oriented external electric field hypothesis.
Supporting Information
The authors have cited additional references within the Supporting Information.
Author Contributions
L. G. and N. A. devised the project and designed the experiments. N. A. carried out calculations, did the experimental work together with J. M. and analyzed the obtained data. M. S. solved and refined the scXRD data. L. G. and N. A. wrote the manuscript.
Acknowledgments
The authors acknowledge M.Sc. Qingqing Luo, M.Sc. Heiko Ruppert and M.Sc. Manuel Schmitt for help in refinement of scXRD structures. This work was supported by Deutsche Forschungsgemeinschaft (grants GR 5007/2-1). Support by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster) is acknowledged. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.