Ortho-Alkoxy-benzamide Directed Formation of a Single Crystalline Hydrogen-bonded Crosslinked Organic Framework and Its Boron Trifluoride Uptake and Catalysis
Fangzhou Li
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorDr. Errui Li
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorDr. Krishanu Samanta
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorZhaoxi Zheng
Department of Chemistry, Brandeis University, Waltham, MA 02453 USA
Search for more papers by this authorDr. Lianqian Wu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 USA
Search for more papers by this authorAlbert D. Chen
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorProf. Dr. Omar K. Farha
Department of Chemistry, Northwestern University, Evanston, IL 60208 USA
Search for more papers by this authorDr. Richard J. Staples
Department of Chemistry, Michigan State University, East Lancing, MI 48824 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jia Niu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Klaus Schmidt-Rohr
Department of Chemistry, Brandeis University, Waltham, MA 02453 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Chenfeng Ke
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorFangzhou Li
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorDr. Errui Li
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorDr. Krishanu Samanta
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorZhaoxi Zheng
Department of Chemistry, Brandeis University, Waltham, MA 02453 USA
Search for more papers by this authorDr. Lianqian Wu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 USA
Search for more papers by this authorAlbert D. Chen
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorProf. Dr. Omar K. Farha
Department of Chemistry, Northwestern University, Evanston, IL 60208 USA
Search for more papers by this authorDr. Richard J. Staples
Department of Chemistry, Michigan State University, East Lancing, MI 48824 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Jia Niu
Department of Chemistry, Boston College, Chestnut Hill, MA 02467 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Klaus Schmidt-Rohr
Department of Chemistry, Brandeis University, Waltham, MA 02453 USA
Search for more papers by this authorCorresponding Author
Prof. Dr. Chenfeng Ke
Department of Chemistry, Dartmouth College, Hanover, NH 03755 USA
Search for more papers by this authorGraphical Abstract
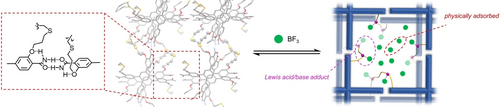
A single-crystalline ortho-alkoxy-benzamide directed hydrogen-bonded crosslinked organic framework (HCOF-50) has been synthesized for BF3 adsorption and demonstrates a record-high capacity of 14.2 mmol/g. The formed HCOF-50 ⋅ BF3 complex showed controlled BF3-releasing for more controlled cationic vinyl ether polymerization.
Abstract
Boron trifluoride (BF3) is a highly corrosive gas widely used in industry. Confining BF3 in porous materials ensures safe and convenient handling and prevents its degradation. Hence, it is highly desired to develop porous materials with high adsorption capacity, high stability, and resistance to BF3 corrosion. Herein, we designed and synthesized a Lewis basic single-crystalline hydrogen-bond crosslinked organic framework (HCOF-50) for BF3 storage and its application in catalysis. Specifically, we introduced self-complementary ortho-alkoxy-benzamide hydrogen-bonding moieties to direct the formation of highly organized hydrogen-bonded networks, which were subsequently photo-crosslinked to generate HCOFs. The HCOF-50 features Lewis basic thioether linkages and electron-rich pore surfaces for BF3 uptake. As a result, HCOF-50 shows a record-high 14.2 mmol/g BF3 uptake capacity. The BF3 uptake in HCOF-50 is reversible, leading to the slow release of BF3. We leveraged this property to reduce the undesirable chain transfer and termination in the cationic polymerization of vinyl ethers. Polymers with higher molecular weights and lower polydispersity were generated compared to those synthesized using BF3 ⋅ Et2O. The elucidation of the structure–property relationship, as provided by the single-crystal X-ray structures, combined with the high BF3 uptake capacity and controlled sorption, highlights the molecular understanding of framework-guest interactions in addressing contemporary challenges.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in CCDC at https://www.ccdc.cam.ac.uk/, reference number 2260614.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202311601-sup-0001-CK1022E.cif1.2 MB | Supporting Information |
| anie202311601-sup-0001-CK1222A.cif1.1 MB | Supporting Information |
| anie202311601-sup-0001-CK722B_auto.cif2.1 MB | Supporting Information |
| anie202311601-sup-0001-exp_485.cif493.8 KB | Supporting Information |
| anie202311601-sup-0001-HCOF-BF3-0.8.cif1.2 MB | Supporting Information |
| anie202311601-sup-0001-misc_information.pdf7.9 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1W. Zhu, X. Zhen, J. Wu, Y. Cheng, J. An, X. Ma, J. Liu, Y. Qin, H. Zhu, J. Xue, X. Jiang, Nat. Commun. 2021, 12, 3957.
- 2
- 2aG. S. Prakash, T. Mathew, E. R. Marinez, P. M. Esteves, G. Rasul, G. A. Olah, J. Org. Chem. 2006, 71, 3952–3958;
- 2bA. Engler, P. A. Kohl, Macromolecules 2020, 53, 1543–1549.
- 3H. Sachdev, M. Strauß, Diamond Relat. Mater. 2000, 9, 614–619.
- 4
- 4aD. Chaudhuri, A. Kumar, I. Rudra, D. D. Sarma, Adv. Mater. 2001, 13, 1548–1551;
- 4bL. Wang, Z. Sofer, P. Šimek, I. Tomandl, M. Pumera, J. Phys. Chem. C 2013, 117, 23251–23257.
- 5V. Jonas, G. Frenking, M. T. Reetz, J. Am. Chem. Soc. 1994, 116, 8741–8753.
- 6J. Cui, Q. Jia, R. Z. Feng, S. S. Liu, T. He, C. Zhang, Org. Lett. 2014, 16, 1442–1445.
- 7G. M. Rusch, G. M. Hoffman, R. F. Mcconnell, W. E. Rinehart, Toxicol. Appl. Pharmacol. 1986, 83, 69–78.
- 8M. M. Lakouraj, M. Mokhtary, Monatsh. Chem. 2009, 140, 53–56.
- 9
- 9aK. Wilson, J. Clark, Chem. Commun. 1998, 2135–2136;
- 9bK. Wilson, D. J. Adams, G. Rothenberg, J. H. Clark, J. Mol. Catal. A 2000, 159, 309–314.
- 10
- 10aP. J. M. Carrott, Carbon 1991, 29, 507–513;
- 10bS. K. Chakrabartty, S. Parkash, N. Berkowitz, Fuel 1976, 55, 270–272.
- 11
- 11aW. P. Fletcher III, A. I. Biaglow, Catal. Lett. 1998, 54, 217–222;
- 11bM. A. Makarova, S. P. Bates, J. Dwyer, J. Am. Chem. Soc. 1995, 117, 11309–11313.
- 12P. W. Siu, J. P. Siegfried, M. H. Weston, P. E. Fuller, W. Morris, C. R. Murdock, W. J. Hoover, R. K. Richardson, S. Rodriguez, O. K. Farha, Inorg. Chem. 2016, 55, 12110–12113.
- 13
- 13aY. Lin, X. Jiang, S. T. Kim, S. B. Alahakoon, X. Hou, Z. Zhang, C. M. Thompson, R. A. Smaldone, C. Ke, J. Am. Chem. Soc. 2017, 139, 7172–7175;
- 13bX. Jiang, X. Cui, A. J. E. Duncan, L. Li, R. P. Hughes, R. J. Staples, E. V. Alexandrov, D. M. Proserpio, Y. Wu, C. Ke, J. Am. Chem. Soc. 2019, 141, 10915–10923.
- 14
- 14aK. Hema, K. M. Sureshan, Acc. Chem. Res. 2019, 52, 3149–3163;
- 14bS. B. Alahakoon, K. Tan, H. Pandey, S. D. Diwakara, G. T. McCandless, D. I. Grinffiel, A. Durand-Silva, T. Thonhauser, R. A. Smaldone, J. Am. Chem. Soc. 2020, 142, 12987–12994.
- 15
- 15aM. Mastalerz, I. M. Oppel, Angew. Chem. Int. Ed. 2012, 51, 5252–5255;
- 15bH. Yamagishi, H. Sato, A. Hori, Y. Sato, R. Matsuda, K. Kato, T. Aida, Science 2018, 361, 1242–1246;
- 15cB. Wang, R. He, L. H. Xie, Z. J. Lin, X. Zhang, J. Wang, H. Huang, Z. Zhang, K. S. Schanze, J. Zhang, S. Xiang, B. Chen, J. Am. Chem. Soc. 2020, 142, 12478–12485;
- 15dP. Cui, E. S. Grape, P. R. Spackman, Y. Wu, R. Clowes, G. M. Day, A. K. Inge, M. A. Little, A. I. Cooper, J. Am. Chem. Soc. 2020, 142, 12743–12750;
- 15eY. Suzuki, M. Gutierrez, S. Tanaka, E. Gomez, N. Tohnai, N. Yasuda, N. Matubayasi, A. Douhal, I. Hisaki, Chem. Sci. 2021, 12, 9607–9618;
- 15fY. Yang, L. Li, R. B. Lin, Y. Ye, Z. Yao, L. Yang, F. Xiang, S. Chen, Z. Zhang, S. Xiang, B. Chen, Nat. Chem. 2021, 13, 933–939;
- 15gC. Zhao, L. Chen, Y. Che, Z. Pang, X. Wu, Y. Lu, H. Liu, G. M. Day, A. I. Cooper, Nat. Commun. 2021, 12, 817;
- 15hH. Zhang, Y. Li, L. Chen, Y. Yang, H. Lin, S. Xiang, B. Chen, Z. Zhang, Chem 2023, 9, 242–252.
- 16R. Liang, J. Samanta, B. Shao, M. Zhang, R. J. Staples, A. D. Chen, M. Tang, Y. Wu, I. Aprahamian, C. Ke, Angew. Chem. Int. Ed. 2021, 60, 23176–23181.
- 17C. B. Aakeröy, B. M. T. Scott, J. Desper, New J. Chem. 2007, 31, 2044–2051.
- 18
- 18aK. Kobayashi, A. Sato, S. Sakamoto, K. Yamaguchi, J. Am. Chem. Soc. 2003, 125, 3035–3045;
- 18bP. Holý, P. Sehnal, M. Tichý, J. Závada, I. Císařová, Tetrahedron: Asymmetry 2003, 14, 245–253;
- 18cM. Seo, J. Park, S. Y. Kim, Org. Biomol. Chem. 2012, 10, 5332–5342.
- 19H. Sigel, R. B. Martin, Chem. Rev. 1982, 82, 385–426.
- 20J. Samanta, Y. Zhang, M. Zhang, A. D. Chen, C. Ke, Acc. Mater. Res. 2022, 3, 1186–1200.
- 21
- 21aX. Yang, S. Martinovic, R. D. Smith, B. Gong, J. Am. Chem. Soc. 2003, 125, 9932–9933;
- 21bY. Zhang, Y. Zhong, A. L. Connor, D. P. Miller, R. Cao, J. Shen, B. Song, E. S. Baker, Q. Tang, S. Pulavarti, R. Liu, Q. Wang, Z. L. Lu, T. Szyperski, H. Zeng, X. Li, R. D. Smith, E. Zurek, J. Zhu, B. Gong, J. Am. Chem. Soc. 2019, 141, 14239–14248.
- 22Deposition numbers 2260614 (for M4Acid), 2260615 (for M3ABM), 2260617 (for M4ABM), and 2260616 (for HCOF-50) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 23
- 23aC. Sun, M. Shen, A. D. Chavez, A. M. Evans, X. Liu, B. Harutyunyan, N. C. Flanders, M. C. Hersam, M. J. Bedzyk, M. Olvera de la Cruz, W. R. Dichtel, Proc. Natl. Acad. Sci. USA 2018, 115, 8883–8888;
- 23bK. Hema, A. Ravi, C. Raju, K. M. Sureshan, Chem. Sci. 2021, 12, 5361–5380;
- 23cM. Zhang, J. Samanta, B. A. Atterberry, R. Staples, A. J. Rossini, C. Ke, Angew. Chem. Int. Ed. 2022, 61, e202214189.
- 24P. Duan, K. Schmidt-Rohr, J. Magn. Reson. 2017, 285, 68–78.
- 25J.-D. Mao, K. Schmidt-Rohr, Environ. Sci. Technol. 2004, 38, 2680–2684.
- 26
- 26aP. Li, Y. He, Y. Zhao, L. Weng, H. Wang, R. Krishna, H. Wu, W. Zhou, M. O'Keeffe, Y. Han, B. Chen, Angew. Chem. Int. Ed. 2015, 54, 574–577;
- 26bY. Yang, H. Zhang, Z. Yuan, J. Q. Wang, F. Xiang, L. Chen, F. Wei, S. Xiang, B. Chen, Z. Zhang, Angew. Chem. Int. Ed. 2022, 61, e202207579;
- 26cY. Sun, J. Wei, Z. Fu, M. Zhang, S. Zhao, G. Xu, C. Li, J. Zhang, T. Zhou, Adv. Mater. 2023, 35, 2208625.
- 27
- 27aP. Tarakeshwar, S. J. Lee, J. Y. Lee, K. S. Kim, J. Phys. Chem. B 1999, 103, 184–191;
- 27bS. J. Grabowski, Struct. Chem. 2017, 28, 1163–1171.
- 28A. E. Bennett, C. M. Rienstra, M. Auger, K. Lakshmi, R. G. Griffin, J. Chem. Phys. 1995, 103, 6951–6958.
- 29E. L. Hahn, Phys. Rev. 1950, 80, 580–594.
- 30B. Fung, A. Khitrin, K. Ermolaev, J. Magn. Reson. 2000, 142, 97–101.
- 31S. Cao, X. Yang, Z. Zhang, J. Wu, B. Chi, H. Chen, J. Yu, S. Feng, Y. Xu, J. Li, Y. Zhang, X. Wang, Y. Wang, Eur. J. Med. Chem. 2022, 230, 114089.
- 32G. M. Sheldrick, Acta Crystallogr. Sect. A 2015, 71, 3–8.
- 33G. M. Sheldrick, Acta Crystallogr. Sect. C 2015, 71, 3–8.
- 34O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Crystallogr. 2009, 42, 339–341.
- 35R. L. Johnson, K. Schmidt-Rohr, J. Magn. Reson. 2014, 239, 44–49.
- 36D. A. Torchia, J. Magn. Reson. (1969–1992) 1978, 30, 613–616.
- 37P. Caravatti, L. Braunschweiler, R. R. Ernst, Chem. Phys. Lett. 1983, 100, 305–310.
- 38A. Bielecki, A. C. Kolbert, H. J. M. De Groot, R. G. Griffin, M. H. Levitt, Adv. Magn. Opt. Reson. 1990, 14, 111–124.
10.1016/B978-0-12-025514-6.50011-3 Google Scholar
- 39J. A. García-Calzón, M. E. Díaz-García, Sens. Actuators B 2007, 123, 1180–1194.