Late-Stage Aryl C−H Bond Cyclopropenylation with Cyclopropenium Cations
Graphical Abstract
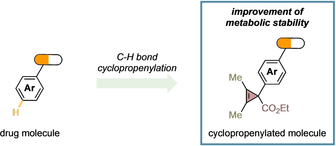
A new late-stage functionalization of complex molecules enables the installation of cyclopropene rings with excellent efficiency, thus reaching an uncharted cyclopropenylated chemical space. The cyclopropene ring generally improves the metabolic stability of the cyclopropenylated drug molecules and serves as a unique scaffold to access broad range of medically-relevant motifs.
Abstract
Herein, we disclose the first regio-, site- and chemoselective late-stage (hetero)aryl C−H bond cyclopropenylation with cyclopropenium cations (CPCs). The process is fast, operationally simple and shows an excellent functional group tolerance in densely-functionalized drug molecules, natural products, agrochemicals and fluorescent dyes. Moreover, we discovered that the installation of the cyclopropene ring in drug molecules could not only be used to shield against metabolic instability but also as a synthetic tool to reach medicinally-relevant sp3-rich scaffolds exploiting the highly-strained nature of the cyclopropene ring with known transformations.
Cyclopropenes are versatile building blocks in organic synthesis that can serve as precursors of cyclopropanes or alternative complex cores.1 They can be found in natural products, and have shown to be metabolically stable and compatible with living systems.2 In fact, they have been used as bioorthogonal chemical reporters in biological environments3 and as plant growth regulators in agriculture and postharvest science.4 However, despite its potential utility as three-dimensional scaffold with four defined exit vectors for pendant functionality, the cyclopropene ring has been largely underexplored in medicinal chemistry (Scheme 1A).5 This is in sharp contrast with other strained ring systems, such as cyclopropane, cyclobutane, oxetane, azetidine and bicyclo[1.1.1]pentane, which are known to improve the physicochemical properties of drug molecules, serve as functional group bioisosteres or provide entry to novel chemical and intellectual property space.6, 7
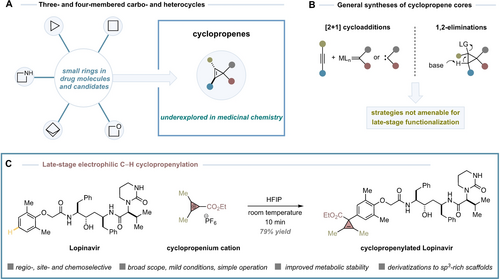
Late-stage C−H bond cyclopropenylation.
The most general syntheses of the cyclopropene ring rely on [2+1] cycloaddition of alkynes with metal-carbenes/free carbenes or 1,2-elimination of cyclopropanes with a strong base (Scheme 1B).1 Both strategies can suffer from functional group intolerance and can be problematic in complex settings, thus limiting their translation and application in drug design. An ideal process to reach an immediate “cyclopropenylated” chemical space potentially useful in drug discovery would rely on the selective installation of cyclopropenyl rings at aryl C−H bonds.8
In 2022, our group reported the first catalytic synthesis of cyclopropenium cations (CPCs)9 using a catalytic carbyne transfer platform with Rh-carbynoids generated from dirhodium complexes and diazomethyl hypervalent iodine reagents.10 The strategy enabled access to a new class of ester-substituted CPCs that reacted with a broad range of carbon- and heteronucleophiles. The nucleophilic attack occurred exclusively at the cyclopropenium carbon atom substituted with the ester group, which was attributed to orbital control. A broad range of nucleophiles were tolerated including aryl-substituted Grignard reagents, boronic acids as well as amines or alcohols, among others.
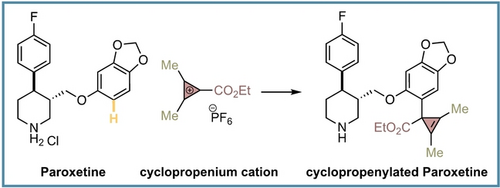
Recently, we questioned whether our ester-substituted CPCs could undergo aryl C−H bond cyclopropenylation reactions in complex settings. The potential utility of this Friedel–Crafts type process would be conditioned to achieving excellent site- and chemoselectivity levels and that could be challenging considering that drug molecules or natural products often display great density of heteronucleophiles and multiple aromatic rings. Such an electrophilic aromatic substitution (SEAr) has been mainly reported between highly-reactive trichlorocyclopropenium cations and simple aromatics.9a, 11 The process provides aryl-substituted cyclopropenium cations upon replacement of the chlorine atom. However, less reactive CPCs substituted with stabilizing groups (amino, alkyl, aryl) tend not to undergo SEAr.9a Herein, we are delighted to present the successful development of this concept for a late-stage (hetero)aryl C−H bond cyclopropenylation reaction as well as its potential utility for drug discovery (Scheme 1C).
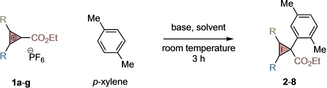
The feasibility of the envisaged electrophilic cyclopropenylation reaction was initially evaluated with p-xylene (2 equiv) and cyclopropenium cation 1 a (1 equiv). We hypothesized that the solvent could play an important role considering the ionic nature of the CPC and SEAr processes. Initially, we tested organic solvents with low polarity such as CH2Cl2 at room temperature. Despite the fact that CH2Cl2 was previously successful with strong nucleophiles,10a it provided only traces of the desired cyclopropene product 2 and instead decomposition of 1 a was observed (Table 1, entry 1). We later observed that non-protic polar solvents such as DMF or CH3CN (entry 2) or protic polar solvents with low acidity such as MeOH were ineffective (entry 3). However, 2,2,2-trifluoroethanol and HFIP provided promising yields of 2 (entries 4,5; 16–20 % yields).12 Unfortunately, further optimization for p-xylene did not afford better yields with 1 a.
|
|||||
Entry |
CPC 1 |
Base |
Solvent |
Product |
Yield [%][b] |
|---|---|---|---|---|---|
1 |
1 a |
– |
CH2Cl2 |
2 |
traces |
2 |
1 a |
– |
DMF, CH3CN CH3NO2 |
2 |
0 |
3 |
1 a |
– |
CH3OH |
2 |
0 |
4 |
1 a |
– |
CF3CH2OH |
2 |
16 |
5 |
1 a |
– |
HFIP |
2 |
20 |
6 |
1 b |
– |
HFIP |
3 |
46 |
7 |
1 b |
CsF |
HFIP |
3 |
92 |
8 |
1 b |
LiF |
HFIP |
3 |
30 |
9 |
1 b |
NaF |
HFIP |
3 |
22 |
10 |
1 b |
Na2CO3 |
HFIP |
3 |
68 |
11 |
1 b |
CsF |
HFIP |
3 |
84[c,d] |
12 |
1 c |
CsF |
HFIP |
4 |
77[c] |
13 |
1 d |
CsF |
HFIP |
5 |
47[c] |
14 |
1 e |
CsF |
HFIP |
6 |
47[c] |
15 |
1 f |
CsF |
HFIP |
7 |
44[c] |
16 |
1 g |
CsF |
HFIP |
8 |
67[c] |
|
|||||
- [a] CPC 1 (0.05–0.12 mmol), p-xylene (0.10–0.30 mmol) and base (0.075–0.1 mmol), solvent (1–2 mL). [b] 1H NMR yields using dibromomethane as internal standard. [c] Yield of isolated pure product. [d] p-Xylene was used as limiting reagent. See Supporting Information for details.
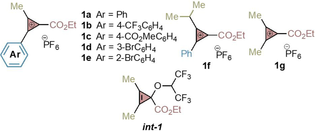
Later, we hypothesized that a more electrophilic CPC should, in principle, provide better efficiency and indeed we were glad to see that CPC 1 b substituted with a 4-CF3-C6H4 group provided the desired cyclopropenyl derivative 3 in 46 % yield (entry 6). Considering that HPF6 was liberated in the reaction, we performed an exploration of additives as proton abstractors and found that CsF enabled formation of 3 in 92 % yield (entry 7). Less soluble LiF or NaF bases gave poor yields (entries 8,9), however, Na2CO3 provided good efficiency (entry 10). Gratifyingly, the reaction also worked well when using p-xylene as limiting reagent (entry 11). Alternative class of CPCs substituted in para (1 c), meta (1 d) and ortho (1 e) positions of the phenyl ring provided cyclopropenes 4–6 in high efficiency (entries 12–14). Finally, CPCs substituted with a secondary alkyl group (entry 15, 1 f) or with two methyl groups were tolerated (entry 16, 1 g).
It was clear that HFIP played an important role in enabling the C−H bond cyclopropenylation reaction. Although it was logical to invoke that HFIP could be stabilizing the ionic species during the SEAr event or forming a solvent-separated ion pair between the cyclopropenium and PF6−, we decided to investigate its role further. To our surprise, we detected the transient formation of int-1 (see Table 1) in reactions conducted with 1 g and p-xylene under the standard reaction conditions monitored by GC-MS. Such an intermediate, generated by the attack of the HFIP to 1 g, could be acting as a cyclopropenium cation reservoir, keeping low concentrations of the reactive CPCs and preventing side reactions. Independent synthesis and reaction of int-1 confirmed its involvement in the process (See Supporting Information for details).
We next turned our attention to evaluate the scope of the C−H bond cyclopropenylation reaction using CPCs 1 a, b, g and an array of simple aromatics, natural products, drug molecules, agrochemicals and fluorescent dyes (Table 2). We observed that C−H bond cyclopropenylations of di-, trisubstituted arenes generally occurred at the least hindered position and most electron-rich aromatic site and provided cyclopropenes 9–19 with high efficiencies (Table 2A). Mono-substituted arenes with strong and moderate electron-donating groups provided the desired cyclopropenes with excellent para-selectivity (20–24). Bulky and weak electron-donating groups were also effective substituents in directing the CPC to the para position (25), however, small groups such as methyl provided the cyclopropene with moderate regioselectivity (26, 27). On the other hand, while arenes substituted with strong or moderate electron-withdrawing groups like nitro or ester were unsuccessful, weak electron-withdrawing groups or simple benzene ring gave modest conversion (28, 29). In addition, we were delighted to observe that naphthalenes (30–32) and heterocycles such as indole (33), pyrrole (34), thiophene (35), furan (36), dihydrobenzofuran (37) and pyridine (38) worked with excellent efficiencies and regioselectivity (Table 2A).
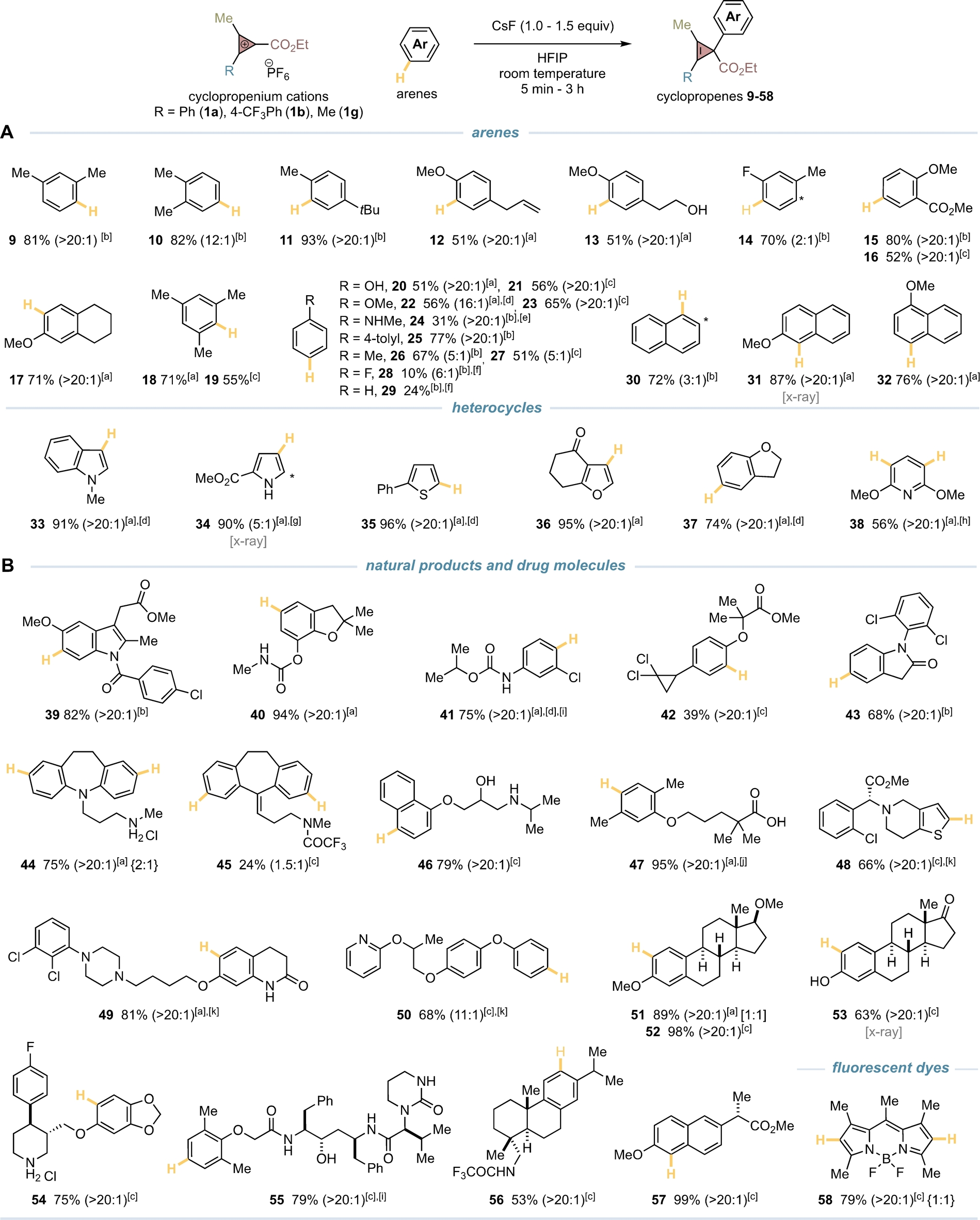
- Reaction conditions: arene (0.10 mmol), CPC 1 (0.12 mmol), CsF (0.10–0.15 mmol), HFIP (2 mL), room temperature. In parenthesis ratio of regioisomers is indicated. In brackets ratio of diastereoisomers is indicated. In curly brackets ratio of mono/bis-cyclopropenylation is indicated. [a] 1 a was used. [b] 1 b was used. [c] 1 g was used. [d] 2,2,2-trifluoroethanol was used as solvent. [e] N-Boc- N -methylaniline was used. [f] Arene was used as cosolvent with HFIP in 3 : 7 ratio. [g] Conducted at 0 °C. [h] Exclusive bis-cyclopropenylation was observed. [i] Conducted without CsF. [j] Starting material with free carboxylic acid was used, for purification purpose the final acid was treated with TMSCHN2 to provide methyl ester derivative 47. [k] HBF4 (50–55 % w/w solution in Et2O) was added.
After this, we demonstrated that our C−H bond cyclopropenylation reaction was effective in complex settings (39–58) (Table 2B). The CPC preference to react chemoselectively at aryl C−H bonds with excellent positional selectivity over other functionalities such as carboxylic acids, esters, ketones, amides, alcohols, alkenes, ureas, ethers, anilines, or hindered secondary amines was striking.13 Less sterically-hindered amines, as well as pyridines were not tolerated, however, the use of the hydrochloric salt derivatives (44, 54) or the addition of Et2O ⋅ HBF4 beforehand (48–50) provided the desired “cyclopropenylated” drug molecules with high efficiency. In addition, we demonstrated our protocol was amenable for the functionalization of BODIPY dyes (58) and could be scaled up to 1.5 gram under air without the need of chromatography (51, 52).14 However, no diastereoselectivity was observed when using 1 a and enantiopure molecules (51, diastereomeric ratio=1 : 1).
To increase the synthetic potential of our C−H bond cyclopropenylation process, we took advantage of the broad and diverse variety of known transformations of cyclopropenes (Table 3).1 We demonstrated that the Pd-catalyzed hydrogenation of 50 and Pauson–Khand reaction of 52 with hexacarbonyldicobalt(1-hexyne) complex were amenable to the selective construction of tetra and hexa-substituted cyclopropane cores (59, 61) (Table 3A). It is well-known that cyclopropenes can undergo metal-catalyzed cycloisomerization reactions involving the cleavage of the cyclopropene C(sp2)−C(sp3) bond, forming an electrophilic vinyl metal-carbene species that can evolve differently depending on the nature of the catalyst.1 We were delighted to observe that while a RhI catalyst was able to deliver tetrasubstituted furan 60, a AuI catalyst provided the formation of indene 62 with excellent stereoselectivity. It is worth noting that the latter example highlights a rare example of a two-step late-stage C−H bond cyclopentannulation.
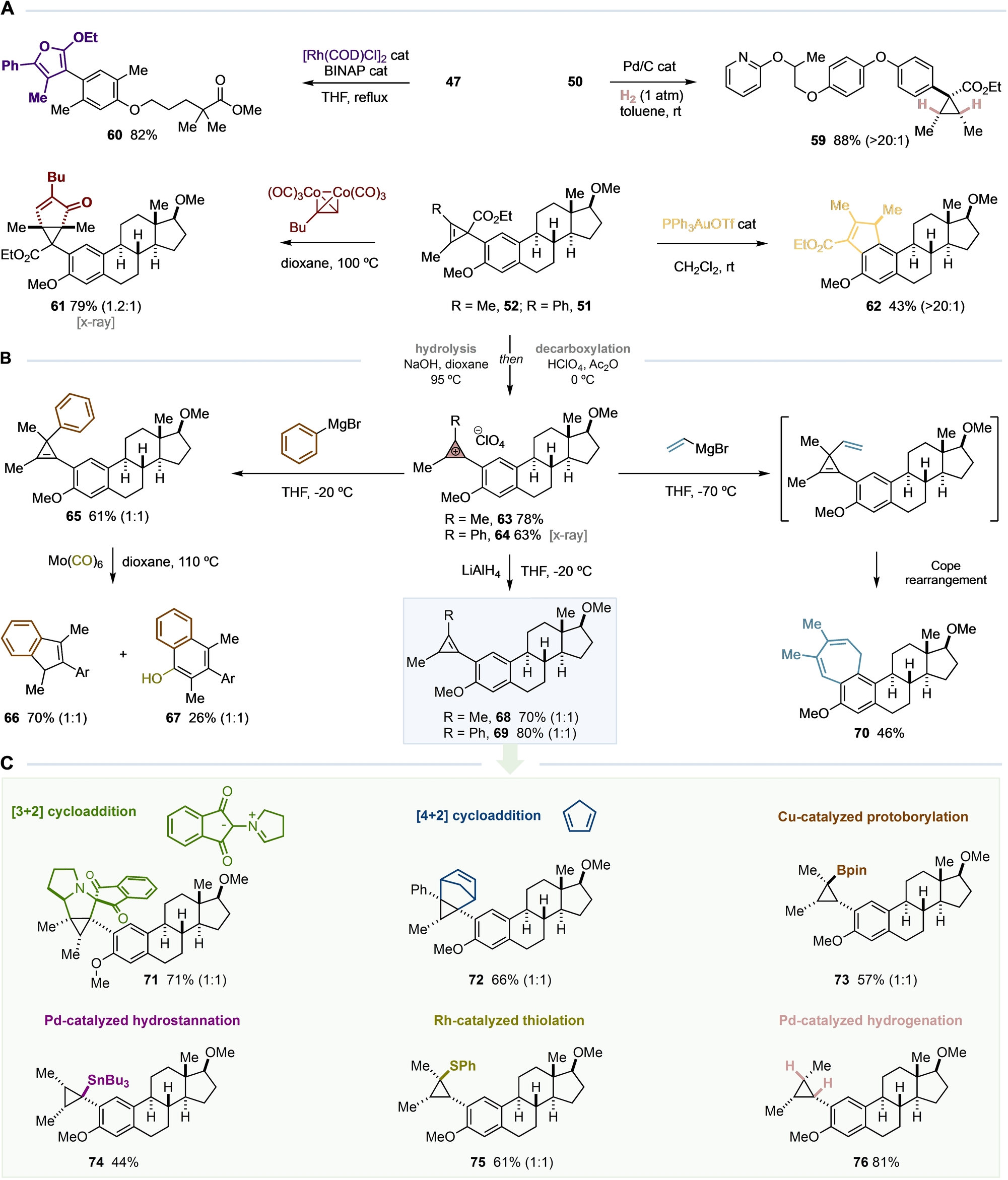
- [a] See the Supporting Information for experimental details. In parentheses the diastereomeric ratio is indicated.
Later, we wondered whether we could expand the synthetic potential of our late-stage C−H bond cyclopropenylation by transforming the cyclopropenyl ester derivative into a new cyclopropenium cation that could undergo nucleophilic additions (Table 3B).15, 16 We were pleased to synthesize new CPCs 63 and 64 as stable solids from 52 and 51 by a two-step protocol involving hydrolysis with NaOH and oxidative decarboxylation with HClO4 in Ac2O. Site-selective attack of phenylmagnesium bromide provided 65, that upon treatment with Mo(CO)6, led to indene 66 and naphthol 67. Reduction with LiAlH4 provided cyclopropenes 68 and 69. In addition, attack of vinylmagnesium bromide to 63 at low temperature provided the formation of cycloheptadiene 70 via a Cope rearrangement of the corresponding vinylcyclopropene intermediate. Finally, we demonstrated that 68 and 69 could undergo cycloaddition reactions (71, 72), protoborylation (73), hydrostannation (74), thiolation (75) and hydrogenation (76) (Table 3C). Notably, these densely functionalized sp3-rich cyclopropane cores are hard to introduce by other approaches and, in the cases of 73 and 74, provide the opportunity for further functionalization by cross-coupling, increasing the potential utility of our cyclopropenylation process.
We demonstrated that our electrophilic late-stage C−H bond cyclopropenylation generally occurred at the most electron-rich aromatic positions of drug molecules, which are common points of metabolic instability through the hydroxylation in the liver, resulting in low in vivo stability and exposure.17 We wondered whether the installation of a cyclopropenyl ring in such position could be used as a tool to shield against metabolic instability of biologically active compounds. To prove our hypothesis, we have measured physicochemical (solubility, Log P) and ADME properties (half-life in rat liver and permeability) of four approved drugs (Propranolol, Clopidogrel, Aripiprazole, Paroxetine) and one pesticide (Pyriproxyfen) together with their corresponding cyclopropenylated derivatives prepared with CPC 1 g (Table 4).
|
|||||
Entry |
Compounds |
Solubility (mM, pH=6.8) |
Log P |
Permeability %FA |
CYP rat (t1/2) (min−1) |
|---|---|---|---|---|---|
1 |
Propranolol |
0.71 |
2.80 |
94.9 |
<1.98 (free amine) |
2 |
46 |
0.75 |
3.65 |
90.3 |
6.11 |
3 |
Clopidogrel |
0.015 |
3.70 |
97.2
|
1.39 |
4 |
48 |
0.001 |
4.70 |
96.6
|
1.39 |
5 |
Aripiprazole |
0.001 |
4.70 |
96.5 |
2.00 |
6 |
49 |
0.013 |
4.70 |
91.4 |
4.29 |
7 |
Pyriproxyfen |
0.001 |
4.70 |
97.6 |
1.39 |
8 |
50 |
0.001 |
4.70 |
87.6 |
3.5 |
9 |
50-OH[b] |
0.001 |
4.70 |
94.8 |
3.15 |
10 |
Paroxetine |
0.75 |
3.60 |
94.1 |
4.9 |
11 |
54 |
0.75 |
4.70 |
93.2 |
14.4 |
- [a] Properties measured from various assay methods (see method details in the SI).
We were delighted to observe an improvement of the metabolic stability in rat liver cells without extensively influencing the solubility or passive penetration properties (Table 4, entries 2, 6, 8, 9, 11).18 Control experiments on alcohol 50-OH, obtained by reduction of the ester moiety on compound 50, also showed an improvement on metabolic stability, confirming the effect of the presence of the 2,3-dimethyl-2-cyclopropene moiety. Moreover, the study of the metabolites through soft-spot identification (SSID method) confirmed that when the substituted position was the one hydroxylated by the liver, the half-life of the compound was improved. The main metabolic soft-spots that were observed in the cyclopropenylated compounds were on the methyl groups of the cyclopropene ring, which is a slower and less efficient oxidation process (see Supporting Information for metabolite structures).
In summary, we have developed a novel late-stage C−H bond functionalization reaction allowing the introduction of cyclopropenyl rings with excellent selectivity and efficiency to a broad range of simple aromatics and complex molecules. We took synthetic advantage of the installation of the cyclopropenyl ring by using well-known transformations, which allowed us to reach molecular diversity currently difficult or even impossible to introduce by late-stage functionalization. Our C−H bond cyclopropenylation may pave the way for cyclopropenes to become commonplace in drug discovery - especially as we have also demonstrated their ability to improve metabolic stability of drug molecules by blocking arene hydroxylation in rat liver cells.
Acknowledgments
European Research Council (ERC-CoG 2019, 865554), Agencia Estatal de Investigación (AEI) of the Ministerio de Ciencia e Innovación (PID2019-104101GB-I00, Severo Ochoa Excellence Accreditation 2020–2023-CEX2019-000925-S), ICIQ Foundation, and the CERCA Programme (Generalitat de Catalunya) are gratefully acknowledged for financial support. We also thank the European Union for a Marie Skłodowska-Curie Individual Fellowship (101032589), Novartis Global Discovery Chemistry funding for visiting scientists as well as Dr. Holly Davis (Novartis) and Dr. Adriana Faraone (ICIQ) for proofreading the manuscript.
Conflict of interest
A provisional patent application has been filed through the Fundació Institut Català D'Investigació Química (ICIQ) based on the results presented here. M. G. Suero, H-.F. Tu, A. Jeandin are listed as inventors.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.