Organic-Platinum Hybrids for Covalent Binding of G-Quadruplexes: Structural Basis and Application to Cancer Immunotherapy
Graphical Abstract
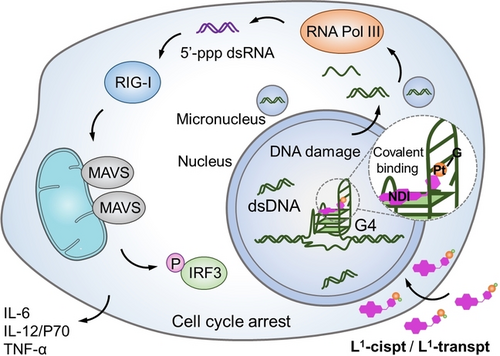
A novel class of G4-targeting organic-platinum hybrids, L1-cispt and L1-transpt, designed to covalently bind to the G4 binding site, could activate the retinoic acid-induced gene I (RIG-I) pathway, induce immunogenic cell death (ICD) and stimulate potent immunotherapy in vivo. This study highlights the importance of the rational combination of specific spatial recognition and covalent binding in G4-targeting drug design.
Abstract
G-quadruplexes (G4s) have been revived as promising therapeutic targets with the development of immunotherapy, but the G4-mediated immune response remains unclear. We designed a novel class of G4-binding organic-platinum hybrids, L1-cispt and L1-transpt, with spatial matching for G4 binding and G4 DNA reactivity for binding site locking. The solution structure of L1-transpt-MYT1L G4 demonstrated the effectiveness of the covalent binding and revealed the covalent binding-guided dynamic balance, accompanied by the destruction of the A5-T17 base pairs to achieve the covalent binding of the platinum unit to N7 of the G6 residue. Furthermore, L1-cispt- and L1-transpt-mediated genomic dysfunction could activate the retinoic acid-induced gene I (RIG-I) pathway and induce immunogenic cell death (ICD). The use of L1-cispt/L1-transpt-treated dying cells as therapeutic vaccines stimulated a robust immune response and effectively inhibited tumor growth in vivo. Our findings highlight the importance of the rational combination of specific spatial recognition and covalent locking in G4-trageting drug design and their potential in immunotherapy.
Conflict of interest
The authors declare no competing interests.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.