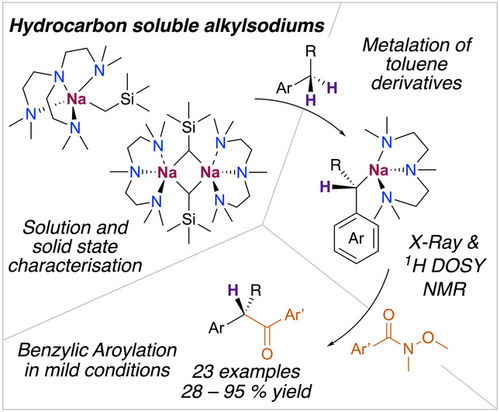
Highly Reactive Hydrocarbon Soluble Alkylsodium Reagents for Benzylic Aroylation of Toluenes using Weinreb Amides
Graphical Abstract
Exploiting coordination effects to prepare hydrocarbon-soluble alkyl organosodium reagents enables the efficient metalation of toluene derivatives under mild and stoichiometric conditions, and their facile conversion into 2-arylacetophenones. Through the isolation of key organometallic intermediates and their study in solution, new mechanistic insights have been gained into these sodium-mediated reactions.
Abstract
Deaggregating the alkyl sodium NaCH2SiMe3 with polydentate nitrogen ligands enables the preparation and characterisation of new, hydrocarbon soluble chelated alkylsodium reagents. Equipped with significantly enhanced metalating power over their organolithium counterparts, these systems can promote controlled sodiation of weakly acidic benzylic C−H bonds from a series of toluene derivatives under mild stoichiometric conditions. This has been demonstrated through the benzylic aroylation of toluenes with Weinreb amides, that delivers a wide range of 2-arylacetophenones in good to excellent yields. Success in isolating and determining the structures of key organometallic intermediates has provided useful mechanistic insight into these new sodium-mediated transformations.
Introduction
Their relative ease of synthesis combined with high Brønsted basicity and solubility in common organic solvents has held organolithium reagents as the gold standard within polar organometallic chemistry for over 100 years, so much so the key reagents are commercially available.1, 2 One of their main synthetic applications is their effectiveness in deprotonative metalation (or lithiation) reactions of aromatic and benzylic substrates, which transforms relatively inert C−H bonds into more reactive C−Li bonds (Figure 1a). Recently, the World has witnessed a groundswell of interest in the need for sustainability and chemistry is no exception, with organometallic chemists now looking beyond lithium for alternatives, due to its spiralling cost and potential instability of supply chains.3-5 While much higher earth-abundant sodium suggests organosodium reagents may be the convenient alternative, to date these heavier congeners have made limited impact in organic synthesis. Among the impediments that have thus far limited the use of these more polar sodium reagents are their poor solubility in hydrocarbon solvents, and their high reactivity, which unlike with their organolithium counterparts, precludes their use in ethereal solvents.6, 7 In addition they exhibit modest functional group tolerance and low thermal stability which not only limits their widespread uses in synthetic organic applications, but also hinders their long-term storage and preparation.8-10 Recent advances in the field have started to address some of these limitations, supporting the vision of a future more sustainable polar organometallic chemistry for functionalising aromatic molecules (Figure 1b).11-14

Examples of reactivity of alkyl-sodium reagents in sodium-halogen exchange and deprotonative sodiation.
For example, Wagner and Miokowski have used sodium dispersion for in situ generation of an alkyl sodium for directed ortho-metalation (DoM) of a small selection of activated arenes.15 More recently Asako and Takai have reported a new method to access arylsodium species via Na-Br exchange using neopentylsodium prepared in situ by reacting neopentylchloride with a sodium dispersion (Figure 1b).16, 17 Knochel has also reported the generation of a hexane-soluble sodium alkyl reagent using a sodium-packed-bed reactor under continuous flow conditions that promotes Na−Br exchange reactions as well as metalation of arenes and alkylarenes (Figure 1b).8, 18 While all promising, it is notable that we are left in the dark as to the constitution of the organometallic intermediates involved in these reactions. Such knowledge would be invaluable on how we could control the high reactivity and overcome the poor solubility of organosodium reagents.
A common strategy to increase the solubility and enhance the reactivity of polar organometallic reagents and s-block metal amides is the use of Lewis donor additives.19 In this regard, Collum has assessed the constitution of sodium amides NaHMDS (HMDS=1,1,1,3,3,3-hexamethyldisilazide) and NaDA (DA=diisopropylamide) in several solvents in the presence of a variety of donor additives and investigated their reactivity in organic transformations such as dehydrohalogenation and metalation.12, 20, 21 Recently we have shown that NaTMP (TMP=2,2′,6,6′-tetramethylpiperidide) combined with the tridentate donor PMDETA (N,N,N′,N′′,N′′-pentamethyldiethylenetriamine) and B(OiPr)3 can promote the regioselective borylation of non-activated substrates, offering in some cases complementary selectivities to those observed using transition-metal catalysed protocols.22 The reactions are driven by the strong metalating power of the NaTMP/PMDETA combination which can also promote catalytic hydrogen isotope exchange of non-activated arenes, including toluene when using C6D6 as the deuterium source.23
Accelerating advancement in this field, here we report the synthesis and characterisation of a hydrocarbon soluble alkyl sodium reagent and its impressive reactivity towards poorly acidic benzylic C−H bonds in forming sodiated benzylic intermediates. We demonstrate the potential of the formed benzyl sodium species in synthetic transformations through a study of the nucleophilic addition to Weinreb amides to access the corresponding synthetically important 2-aryl acetophenones (Figure 1c).24, 25 A key element of the study is trapping several sodium intermediates allowing their identification and those of the parent base and one addition product. We also highlight the ability of benzyl sodium compounds to act as powerful nucleophiles towards unsaturated organic substrates such as CO2, imines and olefins.
Results and Discussion
We began by preparing a new hydrocarbon soluble alkyl sodium reagent. Previously, Knochel showed that (2-ethylhexyl)sodium is soluble in hexane when prepared in flow (see above) although it has moderate stability and needs to be used directly, precluding its storage or further characterisation. Klett and Mulvey also reported that addition of TMEDA (N,N,N′,N′-tetramethylethylenediamine) disrupts the polymeric structure of [{NaCH2SiMe3}4]∞ to give the solvate [(TMEDA)Na(CH2SiMe3)]∞ which is soluble in hexane.26, 27 Inspired by these findings and by the relative stability of this alkyl group which lacks β-hydrogens, we decided to assess the reactivity of NaCH2SiMe3 (1) with the higher dentate donors PMDETA and Me6TREN (tris(N,N-dimethyl-2-aminoethyl)amine) in the hope of forming smaller, more kinetically activated oligomers. While 1 is insoluble in hexane, addition of these high-dentate donors immediately formed colourless solutions which on cooling deposited highly temperature sensitive colourless crystals of [(PMDETA)2Na3(CH2SiMe3)3] (1 a) and [(Me6TREN)Na(CH2SiMe3)] (1 b).
X-ray crystallographic studies established their strikingly different molecular structures (Figure 2). 1 a exhibits a rare trinuclear discrete motif where each alkyl group acts as a bridge between two Na atoms. Adopting an almost linear [Na1…Na2…Na3, 156.12 (7)o] disposition, each Na atom in 1 a adopts a distinct coordination environment with outer Na1 and Na3 being solvated each by a PMDETA molecule. Pseudo-trigonal planar Na2 coordinates to three alkyl groups and forms a medium-long electrostatic (or anagostic) interaction with one Me group of a SiMe3 substituent (Na2-C15…3.241 (10) Å]. Tetracoordinated Na1 only binds to one alkyl group, whereas Na3 achieves pentacoordination by bonding to the remaining two alkyl groups. The Na−C bond lengths in 1 a vary from 2.4672 (77) to 2.7322 (74) Å consistent with the different coordination numbers of the sodium atoms. While this motif is novel in organosodium chemistry, it is reminiscent to the “broken ladder” structures described by Mulvey for lithium amides and later by Strohmann for solvated isopropyl lithium.28, 29 In contrast, 1 b exhibits a unique monomeric arrangement, with pentacoordinated sodium centre solvated by the four N atoms of the Lewis donor and forming a terminal Na−C bond [2.5028 (12) Å]. While monomeric structures are extremely rare in organosodium chemistry, a few examples in the literature have revealed the ability of Me6TREN to favour this type of structural motifs.30-32
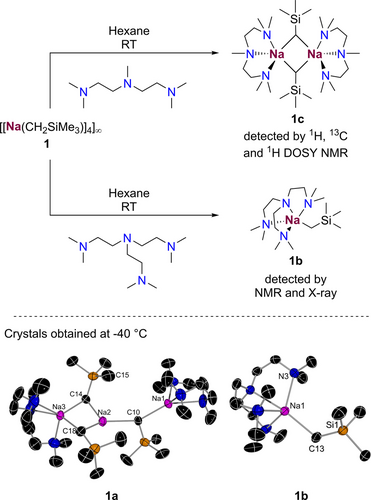
(i) Reactivity studies of [{NaCH2SiMe3}4]∞ towards PMDETA and Me6TREN. (ii) Molecular structures of 1 a and 1 b are shown with 30 % probability displacement ellipsoids. All H atoms have been omitted for clarity.
Benefitting from their high solubility in hydrocarbon solvents 1 a and 1 b were characterised by multinuclear NMR spectroscopy in C6D12 solutions (see Supporting Information for details). 1H DOSY NMR experiments33 on the isolated crystals of 1 a in C6D12 are consistent with the trinuclear structure of 1 a to be retained in solution (estimated molecular weight (MW) 657, 3 % error, see Supporting Information for details). However, it should be noted that 1 a is formed upon low temperature crystallisation of an equimolar mixture of NaCH2SiMe3 and PMDETA in hexane. NMR analysis of an aliquot of this C6D12 solution revealed the presence of a different species in solution, for which our 1H DOSY NMR experiments support a noticeable lower MW to that estimated for 1 a and that we attribute to dimeric [{(PMDETA)Na(CH2SiMe3)}2] (1 c) with a calculated MW of 519 g mol−1 (error −8 %). At room temperature 1 c is highly soluble in hydrocarbon solvents and stability studies in C6D12 solutions have given a half-life of close to 24 h at room temperature at our standard reaction concentration of 0.2 M (see Supporting Information for details). On the other hand, monomeric 1 b proved to be an extremely reactive species which precluded the acquisition of any usable data from the 1H DOSY NMR experiments. Stability studies in C6D12 solutions showed a half-life of under 4 h.
With the characterisation of these remarkably soluble alkyl sodium reagents in hand, we were interested to examine how their aggregation and solution constitution would correlate to their reactivity through sodiation of weakly acidic benzylic C−H bonds. The common aryl solvent toluene is the simplest motif containing a benzylic position and because of its weak acidity (pKa=43 in DMSO),34 its metalation generally requires harsh reaction conditions, with toluene used in a large excess, often times required as the solvent itself.35, 36 Typical metalations of toluene derivatives encounter problems with selectivity when utilising the classical Lochmann-Schlosser superbase (LiCKOR) approach.37, 38 Some limitations can be overcome using the LiNK basic combination developed by O'Shea, where combining LiTMP and KOtBu selective metalation in the benzylic positions of toluenes containing ortho-directing groups can be accomplished.38 More recently Knochel has reported the in situ synthesis of benzyl sodium species using flow conditions (see above) although for toluene or xylenes, the substrate is used neat as a solvent.18 Offering high selectivity and functional group tolerance, Walsh has shown that group 1 metal amides MN(SiMe3)2 (M=Li, Na, K, Cs) can reversibly metalate benzylic C−H bonds, with the fleeting benzyl MCH2Ar intermediates being able to be trapped via electrophilic interception. However high temperatures and long reaction times (110–80 °C, 8–12 h) as well as the use of the benzylic substrate as reaction solvent are required.39-41
For better insight on the metalating capability of NaCH2SiMe3, 1 c was prepared in situ by mixing 1 : 1 equivalents of NaCH2SiMe3 and PMDETA in C6D6 to which was added one equivalent of toluene. 1H NMR monitoring studies revealed that after one hour at room temperature all starting materials have been consumed with the quantitative formation of [(PMDETA)Na(CH2Ph)] (2 a) which exhibits a distinct resonance at 2.53 ppm assigned to the Na-CH2 moiety of a benzyl group. Encouraged by these findings showing the ability of the 1 c combination to efficiently sodiate toluene under mild and stochiometric conditions, and inspired by recent work by Walsh on the deprotonative addition of toluenes to Weinreb amides using the bimetallic LiHMDS/CsF basic combination (see above), we next assessed the nucleophilic power of 2 a towards N-methoxy-N-methylbenzamide (3 a) as a route to access synthetically valuable 2-arylacetophenone 4 a (Table 1).42-45
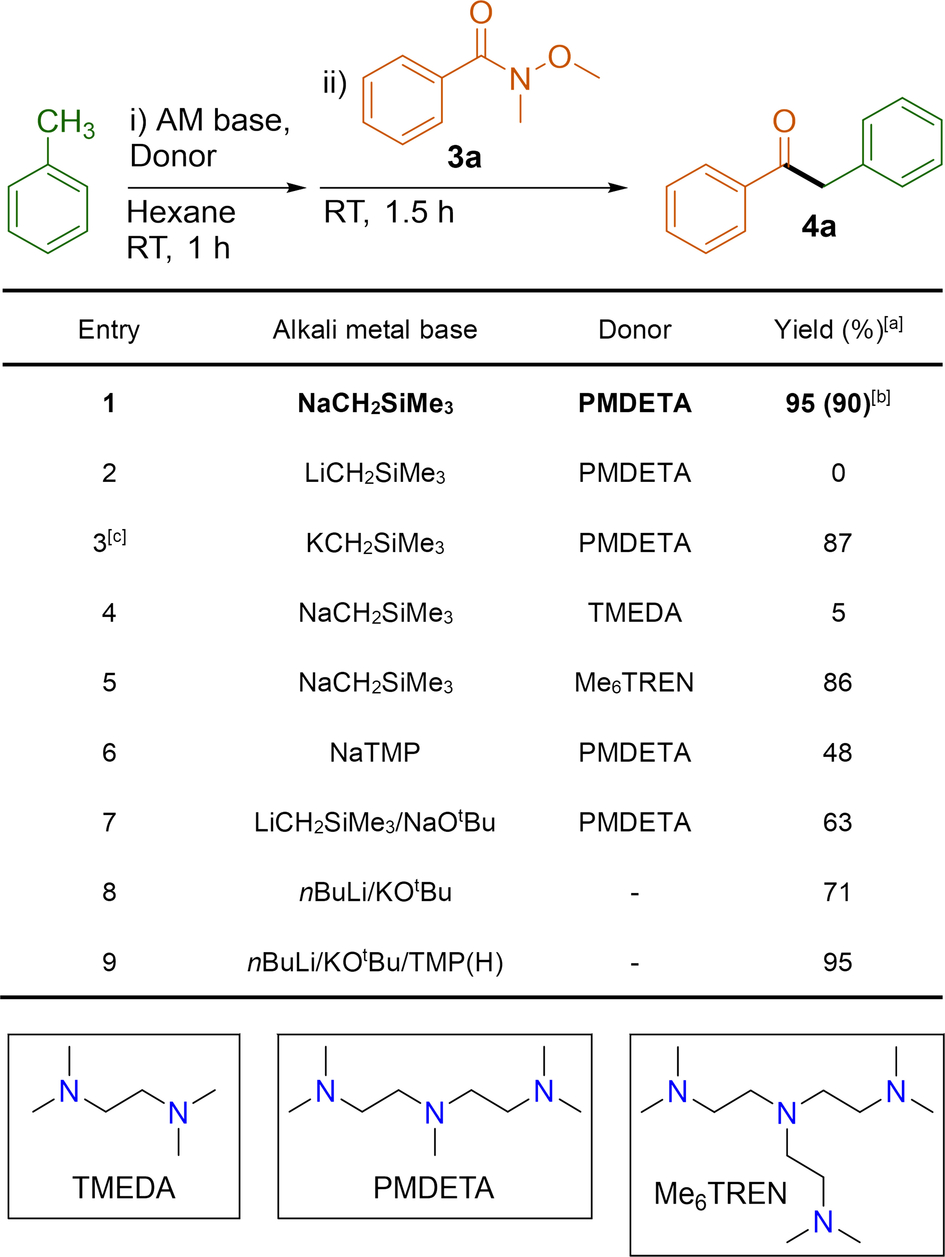
- Conditions: i) Alkali-metal base (1 mmol), donor (1 mmol), toluene (1.5 mmol), hexane (5 mL), room temperature, 1 hour. ii) Weinreb amide (1 mmol) room temperature, 1.5 hours. [a] Yields were determined by 1H NMR spectroscopy using C6Me6 (10 mol %) as internal standard; [b] Isolated yield; [c] 2 h required after addition of Weinreb amide.
To shed light on how the 4 a product is formed, we carried out 1H DOSY NMR studies on [(PMDETA)Na(CH2Ph)] (2 a) in C6D6 solutions (see Supporting Information for details) which suggest that in contrast to its polymeric structure in the solid state, in C6D6 solution it displays a monomeric arrangement (see Supporting Information for details).
Pleasingly we found that the sequential reaction of NaCH2SiMe3/PMDETA with 1.5 equivalents of toluene in hexane followed by addition of 3 a at room temperature furnished 2-phenylacetophenone (4 a) in a 95 % yield (entry 1). These conditions contrast with those required when using LiHMDS and CsF in toluene as a solvent where high temperature, longer reaction times and an excess of base are needed (50–110 °C, 8 h).39 Interestingly a striking alkali-metal effect was discernible. Thus, using LiCH2SiMe3.PMDETA failed to form 4 a that is attributed to diminished basicity of this combination to promote the metalation of toluene under these mild conditions (entry 2). It should be noted that [(PMDETA)Li(CH2SiMe3)] exhibits a monomeric structure in the solid state,46 which is retained in C6D12 solutions (see Supporting Information for 1H DOSY NMR experiments).
Moving down one place in Group 1 to the heavier alkali-metal alkyl KCH2SiMe3 allowed for facile metalation of toluene, however, due to the poor solubility exhibited by the KCH2Ph intermediate a longer reaction time (2 h) was required for the Weinreb amide addition to reach completion (87 %) (entry 3). Finding the appropriate Lewis donor also proved pivotal. Replacing the tridentate donor PMDETA with bidentate TMEDA had a dramatic effect on the efficiency of metalation with NaCH2SiMe3, giving a substantially diminished yield of a mere 5 % (entry 4). The presence of a significant quantity of trimethylsilylmethyl addition to the Weinreb amide (42 %) indicates only a limited percentage of toluene was metalated, with a significant quantity of NaCH2SiMe3 present in solution during Weinreb amide addition. Furthermore, moving to the higher tetradentate donor Me6TREN, to form in situ monomeric 1 b, afforded an excellent yield of 86 %, (entry 5). This shows just how important the correct donor choice is to break the organosodium aggregation and to promote its kinetic activation.47, 48 Bulkier amide NaTMP (entry 6) was less effective at metalating toluene affording 4 a in a 48 % yield. In this case we observe that some of the sodium amide can also react with 3 a causing its partial degradation.49 More classic superbasic conditions were also tested in the model reaction with varying results (entries 7–9). Interestingly we found that using superbasic LiCH2SiMe3/NaOtBu combination (entry 7) in the presence of PMDETA is less effective than employing 1 c furnishing 4 a in a 63 % yield. The LiCKOR base (entry 8) showed a reasonable yield of 71 %, however, the only system with comparable activity to PMDETA⋅NaCH2SiMe3 in the metalation of toluene was O'Shea's LiNK base (entry 9).
In order to assess the selectivity and functional group tolerance of this approach we next extended this study to other substituted toluene substrates (Scheme 1). Simpler alkyl and aryl substituted toluenes (p-xylene, mesitylene, 2-methylnaphthalene, ethylbenzene and diphenylmethane) were converted to the corresponding aryl acetophenones 4 a–f in good to excellent yields (75–95 % yield). It should be noted that for diphenylmethane and ethylbenzene, a solvent mixture of 1 : 1 benzene/hexane was necessary for the nucleophilic addition to the Weinreb amide due to the lack of solubility of the corresponding NaCH2Ar.PMDETA intermediates in neat hexane. Methyl-substituted pyridines (2-methylpyridine, 2,6-dimethylpyridine and 3-methylpyridine), were also compatible with this approach furnishing 4 g, 4 h and 4 i in 81, 93 and 59 % yields respectively. For these systems the aryl acetophenones in the reactions with 2-methyl pyridines were detected in an inseparable mixture with their stable enol tautomers.50 We next turned our attention to substrates containing competing groups which can favour directed-ortho metalation over lateral sodiation.38, 51-53 Using fluorotoluenes as substrates at room temperature led to the thermal decomposition of the relevant metalated intermediates. When sodiation was carried out at −78 °C selective ortho-metalation was observed for 4-fluorotoluene in a reasonable yield (4 j, 41 %). Used at −78 °C, 2-fluorotoluene showed only traces of benzylic metalation alongside a complex mixture of products, contrasting with the LiNK base that has been reported to furnish metalated 2-fluorotoluene in good yields.38 Lateral metalation was seen only for fluoroarenes when both ortho position to F atom were blocked using 2,6-dimethylfluorobenzene, which furnished 4 k in a 57 % yield. Substrates containing an ortho-directing methoxy group exhibited varying reactivity: 4-OMe toluene showed selective metalation ortho to the OMe group giving 4 l in 64 % yield. Whereas 2-OMe and 3-OMe could be selectively metalated at their benzylic position to furnish 4 m and 4 n in 73 and 69 % yield respectively. When two benzylic positions were possible for metalation as with 2,5-dimethylanisole, a mixture of both 2- and 5-metalation was detected (4 o). Selective lateral metalation was also observed for 2-NMe2 toluene giving 4 p in 56 %. Poor metalation and thus modest yields were recorded when 2-methylbenzonitrile was used (4 q, 35 %). Similarly, disappointing yields were also seen for the bis-aryl substrates (2-benzylpyridine, 2-phenylacetonitrile and xanthene). In these cases, NMR studies suggest that while 1 c is able to efficiently metalate these substrates, significantly less nucleophilic sodiated intermediates are obtained which were less reactive towards Weinreb amide addition, favouring instead its decomposition, furnishing products 4 r–4 t in low yields (28–42 %).
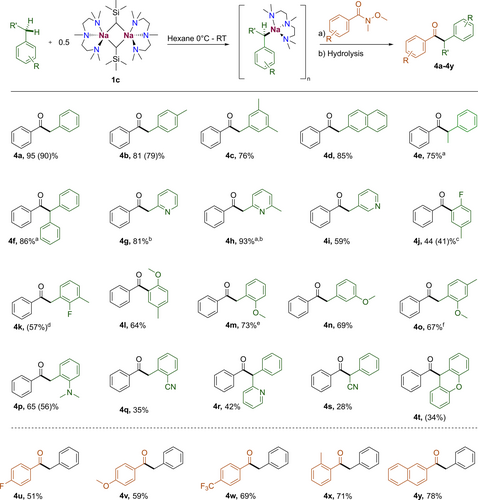
Scope of toluene aroylations. Conditions: a) NaCH2SiMe3 (1 mmol), PMDETA (1 mmol), toluene derivative (1.5 mmol), hexane (5 mL), room temperature, 1 hour. b) Weinreb amide (1 mmol) room temperature, 1.5 hours. Yields were determined by 1H NMR spectroscopy using C6Me6 (10 mol %) as internal standard with all isolated yields in parenthesis. [a] Benzene/hexane solvent system used. [b] Mixture of tautomers. [c] Metalation carried out at −78 °C. [d] Mixture of single and double addition products (57 %/9 %). [e] Metalation carried out at 0 °C. [f] Inseparable mixture of isomers (2 : 1 ratio).
Next, we investigated whether substitution on the Weinreb amide would impact on the outcome of the addition reactions. We saw a tolerance for a para-fluorine substitution to give 4 u, albeit with a moderate yield of 53 %. Electron donating para-methoxy and electron withdrawing para-trifluoromethyl groups were well tolerated, to give 4 v and 4 w in 59 % and 69 % yields respectively. Substitution in the ortho position and the use of the naphthyl derivative were also compatible under our reaction conditions and afforded ketones 4 x and 4 y with yields of 71 % and 78 % respectively.
Aspiring to try and rationalise the findings from these reactivity studies and importantly to correlate them with the constitution of the organometallic intermediates, we successfully isolated the metalation products from the reactions with para-xylene, 2-methoxytoluene and xanthene. Their reaction with equimolar amounts of 1 c in hexane formed yellow and red solids in 54, 64 and 67 % isolated yields respectively that could be recrystallised in benzene/hexane mixtures furnishing [(PMDETA)Na(CH2-4-Me-C6H4)]∞ (2 b), [{(PMDETA)Na(CH2-2-OMe-C6H4)}2] (2 c) and [(PMDETA)Na(C11H9O)] (2 d) (Figure 3 and see Supporting Information for details, 1H NMR analysis of reaction filtrates revealed the formations of 2 b–d occur quantitatively). Compounds 2 b–d were structurally authenticated using X-ray crystallographic studies and their constitution in C6D6 solutions was estimated with the aid of 1H DOSY NMR studies. Demonstrating the structural diversity of sodium benzyl complexes three distinct motifs were found for these PMDETA-solvates. Thus, while 2 b displays a polymeric helical chain motif, 2 c and 2 d adopt discrete dimeric and monomeric arrangements respectively. Exhibiting a structure reminiscent to that reported by Weiss for NaCH2Ph.PMDETA,54 each sodium atom in 2 b achieves pentacoordination by binding to the three PMDETA N atoms, and to two CH2 groups from two different p-xylyl fragments. A close inspection of the Na−C bond lengths revealed that one p-xylyl group bonds to Na via its benzylic C [i.e., Na2−C10, 2.7044 (2) Å], while the remaining group not only coordinates via its benzylic C [Na2−C18, 2.7275 (2) Å], but also forms a medium-long electrostatic π-interaction with the ipso-C of its aromatic ring [Na2−C19, 3.1217 (2) Å].
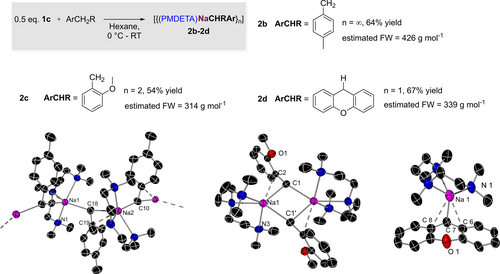
Solid-state structures of sodiated toluene derivatives 2 b–d. Molecular structures shown with 50 % probability displacement ellipsoids. All H atoms have been omitted for clarity.
Anisole derivative 2 c prefers a dimeric structure with a four-membered {NaCNaC} ring core. As with 2 b, each Na is five-coordinate in a distorted trigonal-bipyramidal configuration. Interestingly, the methoxy oxygen atoms play no coordinative role and remain uncoordinated to the sodium atoms. The cation in this dimer is σ bonded to the benzylic position whilst also significantly shifted towards the ipso- carbon of the aryl ring with the angle Na1-C1-C2 at [99.52 (8)°]. The bond length between the benzylic carbon and sodium of 2 c is slightly contracted when compared with that seen in the structure of 2 b, [Na1−C2, 2.528 (1) Å], whereas the sodium-ipso-carbon distance is similar between the two structures [Na1−C2, 3.128 (1) Å].
Compound 2 d was obtained as red crystals via the slow diffusion of hexane into a solution of 2 d in benzene. X-ray crystallographic studies uncovered a monomeric motif for 2 d, with sodium, solvated by PMDETA, sitting about the central C5O ring with a Na1−C7 distance of 2.578 (1) Å with inequivalent bond lengths to the adjacent ipso-C's ([Na1−C8, 3.058 (1) Å] [Na1−C6, 2.873 (1)]). These asymmetric bond lengths are likely the result of steric clash with the bulky PMDETA group positioned directly above one of the two aryl rings. The preferred η3 coordination of the xanthyl group to Na can be attributed to the greater degree of delocalisation of its negative charge between the two aromatic rings, as recently observed by Mulvey and Robertson for alkali-metal complexes containing ditopic aryl methyl anions.55 Such stabilising π-interactions are proving particularly useful in the development of low valent aluminium chemistry.56, 57 We were also interested in the solution state of our metalated intermediates as that would give us a better indication of the aggregation of the sodiated intermediates in our model reaction. 1H DOSY NMR of the crystals of polymeric 2 b in C6D6 revealed a surprising degree of disaggregation, with a calculated MW of 426 g mol−1. This experimental molecular weight could indicate an equilibrium between a monomer (301 g mol−1) and dimer (602 g mol−1) which lies closer to the monomer or alternatively could be a C6D6 solvated monomer (379 g mol−1). 1H DOSY NMR of 2 c in C6D6 also displayed a high level of disaggregation, the calculated MW of 314 g mol−1 giving a strong indication that the metalated species adopts a monomeric structure in C6D6. Unsurprisingly, the solution state structure of 2 d in C6D6 was suggested to be monomeric (339 g mol−1) retaining its discrete structure from the solid-state.
Next, we probed the reaction of [(PMDETA)Na(CH2Ph)] (2 a) with the Weinreb amide 3 a, in an attempt to trap any possible intermediate of this reaction prior to the hydrolysis step. Running the reaction at room temperature in hexane led to the precipitation of a yellow solid that could be recrystallised in toluene at −30 °C producing yellow crystals of sodium enolate [{(PMDETA)Na(OC(=CHPh)Ph)}2] 5 in a 22 % isolated yield. Dimeric 5 consists of a central planar four-membered {NaONaO} ring with Na−O bond lengths in the range 2.3065 (9) to 2.3362 (10) Å. Each Na atom is coordinatively saturated by coordination to a PMDETA molecule. A possible explanation for the formation of 5 is depicted in Figure 4, where initially 2 a undergoes addition to 3 a to form a five-membered ring intermediate I. This type of intermediate has been previously proposed by Collum for the addition of organolithium reagents to Weinreb amides,58 although they are only stable at low temperatures. In our case we could not detect its presence when monitoring the reaction by 1H NMR. Intermediate I could rapidly evolve into 4 a with concomitant elimination of [{(PMDETA)Na(N(OMe)Me)}2] which, in turn, can deprotonate the ketone at the benzylic position to give enolate 5 that we have isolated and identified. It should be noted that formation of related enolate intermediates have been proposed by Walsh as an explanation for the lack of over addition products from the aroylation of poorly nucleophilic diphenylmethanes using N-acyl pyrroles and NaHMDS although in this case harsh reaction conditions (3 eq. of base, 80 °C, 12 h) were employed.59
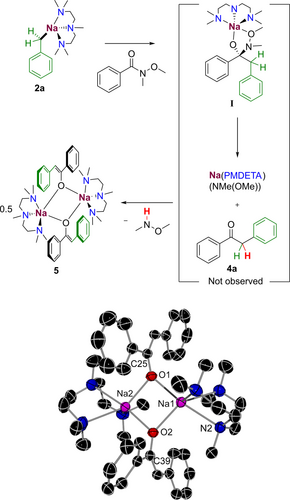
Proposed pathway of sodium enolate 5. Molecular structures are shown with 50 % probability displacement ellipsoids. All H atoms have been omitted for clarity.
We also explored the ability of these new benzyl compounds to undergo insertions of C=O, C=N and C=C bonds (Scheme 2). [(PMDETA)NaCH2Ph)] (2 a) was treated to a CO2 atmosphere to afford upon aqueous work-up phenylacetic acid (6) in a near quantitative yield. Next, 2 a was reacted with N-benzylideneaniline in an attempt to access the α-branched amine N-(1,2-diphenylethyl)aniline 7, the reaction afforded the desired product in an excellent yield of 88 % after purification by column chromatography. 2 a also undergoes addition reactions with simple olefins. Thus, 1,1-diphenylethylene reacted stoichiometrically with benzyl sodium to afford 8 in 83 % yield.
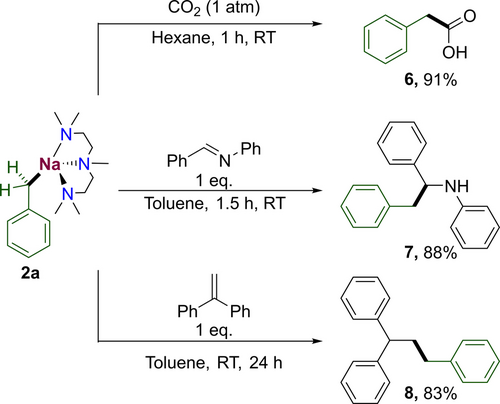
Reactivity studies of 2 a towards other unsaturated organic substrates.
Conclusion
In summary, a facile approach has been developed for the synthesis and isolation of benzyl sodium complexes using a hydrocarbon soluble alkyl sodium reagent [{(PMDETA)Na(CH2SiMe3)}2] (1 c). The high reactivity of these sodiated organometallic species has been demonstrated through the aroylation of metalated toluenes with a Weinreb amide, forming 2-arylacetophenones in good to excellent yields. Combining X-ray crystallographic studies60 with in-depth spectroscopic NMR studies new light has been shed on the constitution of the organometallic intermediates involved in these transformations. Reactivity studies have also shown that benzyl sodium complex [(PMDETA)Na(CH2Ph)] (2 a) can effectively undergo addition reactions towards other unsaturated organic molecules, opening up new ground towards the development of novel sodium-mediated transformations as part of a drive towards more sustainable polar organometallic reagents.
Acknowledgments
We thank the X-ray crystal structure service unit at the University of Bern for measuring, solving, refining, and summarising the structure of compounds 1 a, 1 b, 2 b, 2 c, 2 d and 5. Swiss National Science Foundation (SNF) (projects numbers 206021_177033, 206021_198127 and 188573) and University of Bern are acknowledged for the funding of this research. A. T. also acknowledges the Fundación Ramón Areces for the award of a postdoctoral fellowship. Our thanks are extended to Professor Robert E. Mulvey at the University of Strathclyde for insightful discussions. Open Access funding provided by Universität Bern.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.