Circular Upcycling of Bottlebrush Thermosets
Dr. Daixuan Zhang
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Foad Vashahi
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Erfan Dashtimoghadam
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorDr. Xiaobo Hu
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorClaire J. Wang
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorJessica Garcia
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorAleksandra V. Bystrova
A.N.Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Vavilova St. 28, Moscow, 119334 Russian Federation
Search for more papers by this authorDr. Mohammad Vatankhah-Varnoosfaderani
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorProf. Frank A. Leibfarth
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorCorresponding Author
Prof. Sergei S. Sheiko
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorDr. Daixuan Zhang
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Foad Vashahi
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
These authors contributed equally to this work.
Search for more papers by this authorDr. Erfan Dashtimoghadam
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorDr. Xiaobo Hu
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorClaire J. Wang
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorJessica Garcia
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorAleksandra V. Bystrova
A.N.Nesmeyanov Institute of Organoelement Compounds of Russian Academy of Sciences, Vavilova St. 28, Moscow, 119334 Russian Federation
Search for more papers by this authorDr. Mohammad Vatankhah-Varnoosfaderani
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorProf. Frank A. Leibfarth
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorCorresponding Author
Prof. Sergei S. Sheiko
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599 USA
Search for more papers by this authorGraphical Abstract
Abstract
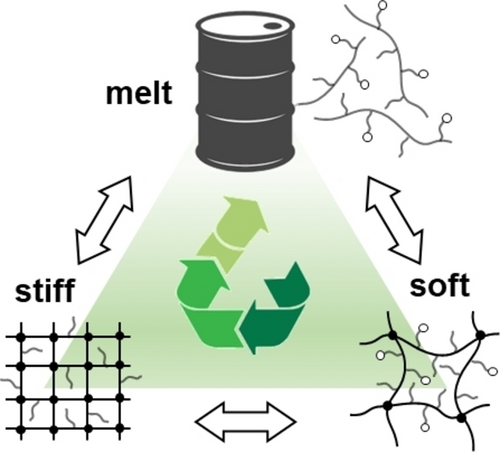
The inability to re-process thermosets hinders their utility and sustainability. An ideal material should combine closed-loop recycling and upcycling capabilities. This trait is realized in polydimethylsiloxane bottlebrush networks using thermoreversible Diels–Alder cycloadditions to enable both reversible disassembly into a polymer melt and on-demand reconfiguration to an elastomer of either lower or higher stiffness. The crosslink density was tuned by loading the functionalized networks with a controlled fraction of dormant crosslinkers and crosslinker scavengers, such as furan-capped bis-maleimide and anthracene, respectively. The resulting modulus variations precisely followed the stoichiometry of activated furan and maleimide moieties, demonstrating the lack of side reactions during reprocessing. The presented circularity concept is independent from the backbone or side chain chemistry, making it potentially applicable to a wide range of brush-like polymers.
Conflict of interest
The authors declare no conflict of interest
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202217941-sup-0001-misc_information.pdf1.3 MB | Supporting Information |
| anie202217941-sup-0001-SI_Video_1.mp47.4 MB | Supporting Information |
| anie202217941-sup-0001-SI_Video_2.mp46.6 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. R. Christensen, A. M. Scheuermann, K. E. Loeffler, B. A. Helms, Nat. Chem. 2019, 11, 442–448.
- 2M. A. Borden, F. A. Leibfarth, Nat. Chem. 2021, 13, 930–932.
- 3M. Häußler, M. Eck, D. Rothauer, S. Mecking, Nature 2021, 590, 423–427.
- 4L. S. T. J. Korley, T. H. Epps, B. A. Helms, A. J. Ryan, Science 2021, 373, 66–69.
- 5B. D. Vogt, K. K. Stokes, S. K. Kumar, ACS Appl. Polym. Mater. 2021, 3, 4325–4346.
- 6K. Ragaert, L. Delva, K. Van Geem, Waste Manage. 2017, 69, 24–58.
- 7J. M. Eagan, J. Xu, R. Di Girolamo, C. M. Thurber, C. W. Macosko, A. M. La Pointe, F. S. Bates, G. W. Coates, Science 2017, 355, 814–816.
- 8A. L. Kocen, S. Cui, T.-W. Lin, A. M. LaPointe, G. W. Coates, J. Am. Chem. Soc. 2022, 144, 12613–12618.
- 9C. Vadenbo, S. Hellweg, T. F. Astrup, J. Ind. Ecol. 2017, 21, 1078–1089.
- 10S. R. Nicholson, J. E. Rorrer, A. Singh, M. O. Konev, N. A. Rorrer, A. C. Carpenter, A. J. Jacobsen, Y. Román-Leshkov, G. T. Beckham, Annu. Rev. Chem. Biomol. Eng. 2022, 13, 301–324.
- 11G. Xu, Q. Wang, Green Chem. 2022, 24, 2321–2346.
- 12Y. Liu, Z. Yu, B. Wang, L. Pengyun, J. Zhu, S. Ma, Green Chem. 2022, 24, 5691–5807.
- 13C. J. Kloxin, C. N. Bowman, Chem. Soc. Rev. 2013, 42, 7161–7173.
- 14S. Oh, E. E. Stache, J. Am. Chem. Soc. 2022, 144, 5745–5749.
- 15T. Tan, W. Wang, K. Zhang, Z. Zhan, W. Deng, Q. Zhang, Y. Wang, ChemSusChem 2022, 15, e202200522.
- 16C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y.-X. Chen, F. A. Leibfarth, H. Sardon, Nature 2022, 603, 803–814.
- 17T. J. Fazekas, J. W. Alty, E. K. Neidhart, A. S. Miller, F. A. Leibfarth, E. J. Alexanian, Science 2022, 375, 545–550.
- 18M. Olszewski, L. Li, G. Xie, A. Keith, S. S. Sheiko, K. Matyjaszewski, J. Polym. Sci. Part A 2019, 57, 2426–2435.
- 19C. M. Plummer, L. Li, Y. Chen, Polym. Chem. 2020, 11, 6862–6872.
- 20M. L. Lepage, C. Simhadri, C. Liu, M. Takaffoli, L. Bi, B. Crawford, A. S. Milani, J. E. Wulff, Science 2019, 366, 875–878.
- 21J. B. Williamson, S. E. Lewis, R. R. Johnson, I. M. Manning, F. A. Leibfarth, Angew. Chem. Int. Ed. 2019, 58, 8654–8668; Angew. Chem. 2019, 131, 8746–8761.
- 22J. B. Williamson, C. G. Na, R. R. Johnson, W. F. M. Daniel, E. J. Alexanian, F. A. Leibfarth, J. Am. Chem. Soc. 2019, 141, 12815–12823.
- 23S. E. Lewis, B. E. Wilhelmy, F. A. Leibfarth, Chem. Sci. 2019, 10, 6270–6277.
- 24S. Ügdüler, K. M. Van Geem, M. Roosen, E. I. P. Delbeke, S. De Meester, Waste Manage. 2020, 104, 148–182.
- 25T. W. Walker, N. Frelka, Z. Shen, A. K. Chew, J. Banick, S. Grey, M. S. Kim, J. A. Dumesic, R. C. Van Lehn, G. W. Huber, Sci. Adv. 2020, 6, eaba7599.
- 26X. Liu, M. Hong, L. Falivene, L. Cavallo, E. Y. X. Chen, Macromolecules 2019, 52, 4570–4578.
- 27B. R. Elling, W. R. Dichtel, ACS Cent. Sci. 2020, 6, 1488–1496.
- 28M. Röttger, T. Domenech, R. Van Der Weegen, A. Breuillac, R. Nicolaÿ, L. Leibler, Science 2017, 356, 62–65.
- 29D. J. Fortman, J. P. Brutman, C. J. Cramer, M. A. Hillmyer, W. R. Dichtel, J. Am. Chem. Soc. 2015, 137, 14019–14022.
- 30T. Rogge, N. Kaplaneris, N. Chatani, J. Kim, S. Chang, B. Punji, L. L. Schafer, D. G. Musaev, J. Wencel-Delord, C. A. Roberts, R. Sarpong, Z. E. Wilson, M. A. Brimble, M. J. Johansson, L. Ackermann, Nat. Rev. Methods Primers 2021, 1, 43.
- 31N. Vora, P. R. Christensen, J. Demarteau, N. R. Baral, J. D. Keasling, B. A. Helms, C. D. Scown, Sci. Adv. 2021, 7, eabf0187.
- 32G. M. Scheutz, J. J. Lessard, M. B. Sims, B. S. Sumerlin, J. Am. Chem. Soc. 2019, 141, 16181–16196.
- 33M. J. Webber, M. W. Tibbitt, Nat. Rev. Mater. 2022, 7, 541–556.
- 34J. Demarteau, A. R. Epstein, P. R. Christensen, M. Abubekerov, H. Wang, S. J. Teat, T. J. Seguin, C. W. Chan, C. D. Scown, T. P. Russell, J. D. Keasling, K. A. Persson, B. A. Helms, Sci. Adv. 2022, 8, abp8823.
- 35M. Vatankhah-Varnosfaderani, A. N. Keith, Y. Cong, H. Liang, M. Rosenthal, M. Sztucki, C. Clair, S. Magonov, D. A. Ivanov, A. V. Dobrynin, S. S. Sheiko, Science 2018, 359, 1509–1513.
- 36C. Clair, A. Lallam, M. Rosenthal, M. Sztucki, M. Vatankhah-Varnosfaderani, A. N. Keith, Y. Cong, H. Liang, A. V. Dobrynin, S. S. Sheiko, D. A. Ivanov, ACS Macro Lett. 2019, 8, 530–534.
- 37J. M. Sarapas, E. P. Chan, E. M. Rettner, K. L. Beers, Macromolecules 2018, 51, 2359–2366.
- 38S. S. Sheiko, F. Vashahi, B. J. Morgan, M. Maw, E. Dashtimoghadam, F. Fahimipour, M. Jacobs, A. N. Keith, M. Vatankhah-Varnosfaderani, A. V. Dobrynin, ACS Cent. Sci. 2022, 8, 845–852.
- 39B. B. Patel, D. J. Walsh, D. H. Kim, J. Kwok, B. Lee, D. Guironnet, Y. Diao, Sci. Adv. 2020, 6, 7202–7212.
- 40F. Vashahi, M. R. Martinez, E. Dashtimoghadam, F. Fahimipour, A. N. Keith, E. A. Bersenev, D. A. Ivanov, E. B. Zhulina, P. Popryadukhin, K. Matyjaszewski, M. Vatankhah-Varnosfaderani, S. S. Sheiko, Sci. Adv. 2022, 8, abm2469.
- 41E. Dashtimoghadam, F. Fahimipour, A. N. Keith, F. Vashahi, P. Popryadukhin, M. Vatankhah-Varnosfaderani, S. S. Sheiko, Nat. Commun. 2021, 12, 3961.
- 42R. Xie, S. Mukherjee, A. E. Levi, V. G. Reynolds, H. Wang, M. L. Chabinyc, C. M. Bates, Sci. Adv. 2020, 6, eabc6900.
- 43C. Choi, J. L. Self, Y. Okayama, A. E. Levi, M. Gerst, J. C. Speros, C. J. Hawker, J. Read De Alaniz, C. M. Bates, J. Am. Chem. Soc. 2021, 143, 9866–9871.
- 44E. H. Discekici, A. H. St. Amant, S. N. Nguyen, I. H. Lee, C. J. Hawker, J. Read De Alaniz, J. Am. Chem. Soc. 2018, 140, 5009–5013.
- 45A. P. Bapat, J. G. Ray, D. A. Savin, E. A. Hoff, D. L. Patton, B. S. Sumerlin, Polym. Chem. 2012, 3, 3112–3120.
- 46E. M. Foster, E. E. Lensmeyer, B. Zhang, P. Chakma, J. A. Flum, J. J. Via, J. L. Sparks, D. Konkolewicz, ACS Macro Lett. 2017, 6, 495–499.
- 47S. Terryn, J. Brancart, E. Roels, R. Verhelle, A. Safaei, A. Cuvellier, B. Vanderborght, G. Van Assche, Macromolecules 2022, 55, 5497–5513.
- 48K. J. Luo, L. B. Huang, Y. Wang, J. R. Yu, J. Zhu, Z. M. Hu, Chin. J. Polym. Sci. 2020, 38, 268–277.
- 49H. Chang, M. S. Kim, G. W. Huber, J. A. Dumesic, Green Chem. 2021, 23, 9479–9488.
- 50X. Kuang, G. Liu, X. Dong, X. Liu, J. Xu, D. Wang, J. Polym. Sci. Part A 2015, 53, 2094–2103.
- 51L. M. Polgar, M. Van Duin, A. A. Broekhuis, F. Picchioni, Macromolecules 2015, 48, 7096–7105.
- 52Q. Zhou, Z. Sang, K. K. Rajagopalan, Y. Sliozberg, F. Gardea, S. A. Sukhishvili, Macromolecules 2021, 54, 10510–10519.