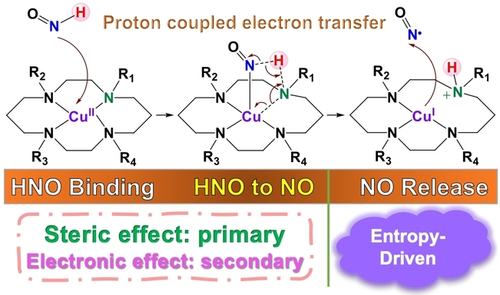
Mechanistic Origin of Favorable Substituent Effects in Excellent Cu Cyclam Based HNO Sensors
Graphical Abstract
HNO has broad chemical and biomedical properties and Cu cyclam is a useful platform to make excellent HNO sensors including imaging agents. A computational study reproduced their various experimental reactivities and revealed subtle and sometimes unexpected interwoven effects of steric and electronic factors, which offer the first mechanistic understanding of substituent effects for excellent HNO sensors to facilitate future development.
Abstract
HNO has broad chemical and biomedical properties. Metal complexes and derivatives are widely used to make excellent HNO sensors. However, their favorable mechanistic origins are largely unknown. Cu cyclam is a useful platform to make excellent HNO sensors including imaging agents. A quantum chemical study of Cu cyclams with various substitutions was performed, which reproduced diverse experimental reactivities. Structural, electronic, and energetic profiles along reaction pathways show the importance of HNO binding and a proton-coupled electron transfer mechanism for HNO reaction. Results reveal that steric effect is primary and electronic factor is secondary (if the redox potential is sufficient), but their interwoven effects can lead to unexpected reactivity, which looks mysterious experimentally but can be explained computationally. This work suggests rational substituent design ideas and recommends a theoretical study of a new design to save time and cost due to its subtle effect.
Introduction
HNO confers vasoprotective effects, increases myocardial contractility, and inhibits platelet aggregation.1 HNO also exerts beneficial effects on ischemia-reperfusion injury,2 cardiovascular diseases,3 anti-alcoholism,4 and antioxidant activity.5 Sensitive HNO detection methods are critical to help unveil its intriguing biomedical functions. However, most HNO detection methods, though useful in many scientific studies, are indirect or inconvenient for in vivo uses.1a, 6 Recently, a number of reaction-based HNO sensors have been developed, utilizing unique structural features such as metals,7 phosphines,8 thiols,9 and ester,10 many of which can be used in vivo.
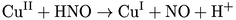 (1)
(1)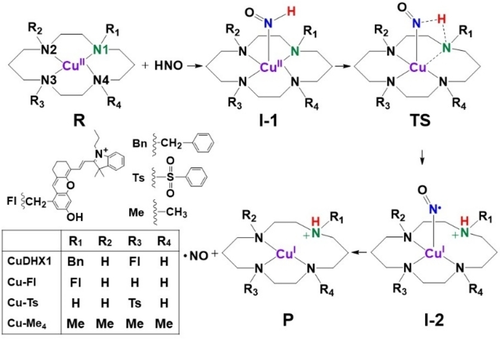
Schematic pathways of HNO reactions with Cu cyclam complexes. Bn=benzyl; Fl=fluorophore; Me=methyl; Ts=tosyl.
In contrast with numerous experimental advances in developing metal-based HNO sensors, there is only one computational reaction mechanism study of such probes,11 which, however, is for the first metal-based HNO sensor with a non-planar Cu coordination environment and mechanism similar to Cu,Zn-superoxide dismutase (CuZnSOD).12 Therefore, we performed a quantum chemical computational study of four Cu cyclam complexes in Scheme 1 to elucidate their unknown substituent effects on the complete reaction pathway: from HNO binding to form the first intermediate (R+HNO→I-1), through HNO conversion (I-1→TS, transition state →I-2) to yield the second intermediate, then NO releasing for final product formation (I-2→P+NO). This work not only reproduced their distinctive experimental HNO reactivities, but more importantly provided previously unknown mechanistic origins of the substituent effects in excellent HNO sensors.
Results and Discussion
We first studied these Cu cyclam complexes to understand the conformation effect, using the same computational method which recently enabled accurate descriptions of HNO reactivities in metalloproteins and model systems.11-13 It is known that Cu cyclam complexes commonly exist in the trans-III form (a square planar structure with the four nitrogen substituents R1/R4 and R2/R3 pointing above and below the plane respectively), or the trans-I form (another square planar structure featuring all four nitrogen substituents pointing above the plane),14 see Scheme 2.
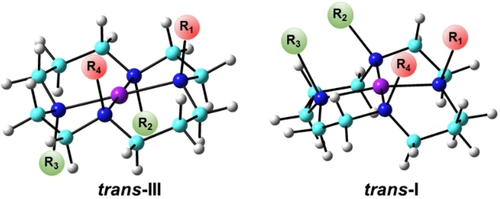
Cu cyclam trans-III and trans-I conformations. Atom color scheme: C- cyan, N- blue, H– grey, Cu- purple.
Among the four Cu systems in Scheme 1, the latter two have crystal structures (each has two triflate OTf− counterions): [(Cu−Ts)(OTf)2] and [(Cu−Me4)(OTf)2]. Energies of the optimized structures show that both prefer the trans-III conformation (see Table S1) as found experimentally,7k and the predicted Cu−N coordination bond lengths (see Table S2) have a mean absolute deviation (MAD) of 0.029 Å and a mean percentage deviation (MPD) of only 1.4 % (even the three loosely coordinated Cu−OTf distances of 2.6 Å were reproduced with ≈5 % error). These results further support the accuracy of the used computational method.
Interestingly, additional calculations show that the trans-I conformation is slightly more favorable than the trans-III form for Cu−Me4 alone (without the counterions) and two conformations are of comparable energies (≈0.5 kcal mol−1 difference) in the case of Cu−Ts itself, Table S1. Thus, the preferred conformation may change in the absence of an axial ligand. Since the experimental Cu−OTf bonds are of 2.6–3.9 Å long,7k the OTf ligands are labile and easily dissolved in the experimental aqueous solution.
Accordingly, we then studied 4-coordinate CuDHX1 and Cu−Fl structures without the labile axial ligand in the solution environment. CuDHX1 has almost the same Gibbs free energies (ΔG=0.09 kcal mol−1) for these two conformations, while Cu−Fl is more favorable in trans-III form by ≈2 kcal mol−1, see Table S1.
These results show that both conformations might exist in the solution due to ≲2 kcal mol−1 energy differences for these studied Cu cyclams and the relatively preferred conformation may vary depending on different substituents and axial ligands. These features support flexible conformation changes that may be needed for subsequent HNO binding and reaction.
As shown in equation 1, the HNO reactions with these Cu cyclams involve both proton and electron transfer. Experimental work shows that Cu−Me4 and Cu−Ts with same redox potentials have no and facile reactivities respectively,7k i.e. a dramatic difference indicating that the electron transfer is not through an out-sphere mechanism, but an inner complex - an HNO bound intermediate, which can also facilitate the proton transfer as found in CuZnSOD and its model sensor.11, 12 Therefore, the HNO binding step to form this intermediate is important to be studied for Cu cyclams.
As such, we first examined the HNO bound structures of Cu−Me4 and Cu−Ts with both trans-I and trans-III conformations and with HNO binding to copper on both the same and opposite sides of substituents called cis and trans respectively here. As shown in Figure S3 and Table S3, none of the trans sides can have stable HNO binding as the resultant Cu−N(HNO) distances of all such structures are>4.2 Å due to steric hindrance. For the cis side binding, only trans-I conformation has HNO coordination and the sterically less hindered Cu−Ts has a shorter Cu−N distance (2.426 Å) than Cu−Me4 (2.854 Å), while the trans-III conformation also has no HNO coordination (RCu−N>4.4 Å). These results show that the stable HNO binding could only occur with certain conformations from the cis side. Then, the relatively favorable cis side binding was studied for CuDHX1 and Cu−Fl with both trans-I and trans-III conformations. Interestingly, for these two Cu cyclams, both conformations can coordinate with HNO (RCu−N=2.5–2.9 Å) and the energy differences of<0.5 kcal mol−1 are insignificant, see Table S3. The Cu−N distances show that the less crowded substitution in Cu−Fl than CuDHX1 leads to shorter/stronger HNO binding in each conformation. These data clearly indicate the importance of steric effect of substitution on HNO binding.
Based on the above investigation, for Cu−Ts and Cu−Me4, we then chose the trans-I conformation results in the subsequent HNO reaction studies because only this conformation has the HNO bound intermediate. For CuDHX1 and Cu−Fl, because both conformations have similar stabilities of the HNO bound intermediates, both were studied. However, as shown in sections 5 and 6 in Supporting Information, the reaction pathways with the trans-III conformations are thermodynamically more favorable and kinetically more feasible for both CuDHX1 and Cu−Fl, thus the trans-III conformation results were used here in the subsequent HNO reaction mechanism study.
It is interesting to note that the HNO binding Gibbs free energies of CuDHX1, Cu−Fl, and Cu−Me4 which have EDGs are around 10–13 kcal mol−1, much higher than that for Cu−Ts with an EWG, 6.81 kcal mol−1, see Table 1. The binding enthalpies also show that Cu−Ts has the strongest HNO binding. The Cu−N distance of 2.4 Å in HNO bound intermediate (I-1) in Cu−Ts is accordingly the shortest, see Table 2. This may not be surprising since the EWG can enhance Cu's electron affinity toward the electron-rich substrate HNO. As a result, the bound HNO transfers the most (negative) charge to Cu in Cu−Ts compared to other Cu cyclams and consequently Cu−Ts−I-1 has the least positive Cu charge and the least negative NO charge. This suggests that beyond the steric effect, the electronic effect of a substituent may also play a significant role on HNO binding. In fact, both Cu−Ts and Cu−Fl bear the smallest number of substituents and thus similarly least steric effect, but since Ts group is an EWG, unlike Fl in Cu−Fl, Cu−Ts has a more favorable binding energy than Cu−Fl. With both favorable steric and electronic effects, Cu−Ts has the most stable HNO bound intermediate, see Table 1.
System |
Step |
ΔE |
ΔEZPE[a] |
ΔH |
ΔG |
|---|---|---|---|---|---|
CuDHX1 |
R+HNO→I-1 |
−0.80 |
0.38 |
0.59 |
9.84 |
|
I-1→ I-2 |
−4.84 |
−3.94 |
−3.93 |
−4.01 |
|
I-2→P+NO |
−1.28 |
−2.91 |
−3.26 |
−13.21 |
Cu−Fl |
R+HNO→I-1 |
−1.77 |
−0.09 |
−0.15 |
10.96 |
|
I-1→I-2 |
1.43 |
1.45 |
1.20 |
−0.33 |
|
I-2→P+NO |
−0.68 |
−1.7 |
−1.17 |
−10.88 |
Cu−Ts |
R+HNO→I-1 |
−4.93 |
−3.36 |
−3.42 |
6.81 |
|
I-1→I-2 |
−2.07 |
−1.66 |
−1.67 |
−2.07 |
|
I-2→P+NO |
0.66 |
−0.06 |
0.28 |
−9.69 |
Cu−Me4 |
R+HNO→I-1 |
0.56 |
2.24 |
2.18 |
12.52 |
|
I-1→I-2 |
−6.77 |
−5.91 |
−5.97 |
−5.65 |
|
I-2→P+NO |
2.37 |
0.98 |
1.16 |
−8.64 |
- [a] Zero-point energy corrected electronic energy.
Species |
ΔE [kcal mol−1] |
ΔEZPE [kcal mol−1] |
ΔH [kcal mol−1] |
ΔG [kcal mol−1] |
RCu−N [Å] |
RH−N [Å] |
RH⋅⋅⋅N1 [Å] |
RCu−N1 [Å] |
ραβCu [e] |
ραβNO [e] |
QCu [e] |
QNO [e] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
CuDHX1−I-1 |
0.00 |
0.00 |
0.00 |
0.00 |
2.936 |
1.045 |
4.475 |
2.111 |
0.474 |
0.000 |
1.035 |
−0.216 |
CuDHX1−TS |
21.61 |
20.31 |
19.86 |
20.59 |
2.083 |
1.059 |
1.897 |
3.136 |
0.463 |
0.111 |
0.937 |
−0.217 |
CuDHX1−I-2 |
−4.84 |
−3.94 |
−3.93 |
−4.01 |
2.269 |
2.082 |
1.027 |
3.153 |
−0.047 |
1.062 |
0.630 |
−0.071 |
Cu−Fl−I-1 |
−0.97 |
−0.47 |
−0.74 |
1.12 |
2.650 |
1.046 |
4.231 |
2.133 |
0.500 |
0.001 |
0.981 |
−0.222 |
Cu−Fl−TS |
19.2 |
18.66 |
17.98 |
19.96 |
2.087 |
1.049 |
2.091 |
3.183 |
0.502 |
0.080 |
0.935 |
−0.167 |
Cu−Fl−I-2 |
0.46 |
0.98 |
0.46 |
0.79 |
2.259 |
2.138 |
1.028 |
3.031 |
−0.041 |
1.054 |
0.600 |
−0.056 |
Cu−Ts−I-1 |
−4.13 |
−3.74 |
−4.01 |
−3.03 |
2.426 |
1.044 |
4.341 |
2.064 |
0.521 |
0.003 |
0.935 |
−0.196 |
Cu−Ts−TS |
20.69 |
19.33 |
18.63 |
20.50 |
2.053 |
1.078 |
1.831 |
2.996 |
0.457 |
0.181 |
0.887 |
−0.209 |
Cu−Ts−I-2 |
−6.20 |
−5.40 |
−5.68 |
−5.10 |
2.159 |
2.381 |
1.023 |
3.130 |
−0.020 |
1.030 |
0.561 |
−0.028 |
Cu−Me4−I-1 |
1.36 |
1.86 |
1.59 |
2.68 |
2.854 |
1.048 |
4.133 |
2.107 |
0.461 |
0.000 |
1.117 |
−0.255 |
Cu−Me4−TS |
19.81 |
19.34 |
18.30 |
21.70 |
2.034 |
1.063 |
1.849 |
3.078 |
0.422 |
0.134 |
0.999 |
−0.228 |
Cu−Me4−I-2 |
−5.41 |
−4.05 |
−4.38 |
−2.97 |
2.096 |
2.121 |
1.026 |
3.347 |
−0.126 |
1.206 |
0.729 |
−0.191 |
- [a] Energies are relative to CuDHX1−I-1′s reaction energy in the pathway.
In contrast, Cu−Me4 with the most substituents and thus the highest steric effect plus an unfavorable electronic effect here is associated with the greatest binding energy (ΔG of 12.52 kcal mol−1). In fact, this is the only Cu cyclam studied here possessing a positive binding electronic energy ΔE (see Table 1), which means that even the electronic interaction between HNO and Cu−Me4 is unstable and thus there is no stable HNO bound intermediate for this system. Compared with Cu−Me4 displaying no experimental HNO reactivity, the other three Cu cyclams with HNO reactivities all have negative binding electronic energies (see Table 1). These results again highlight the importance of the formation of a stable HNO bound intermediate for subsequent reaction. In fact, a stable HNO bound intermediate also exists in the CuZnSOD reaction with HNO.12 Although CuDHX1 and Cu−Fl have electron-donating substituents that are electronically unfavorable for HNO binding, they still have HNO bound intermediates due to moderate steric effects from two and one substituents. These results suggest that steric effect is the dominant factor for HNO binding, while electronic effect is secondary and may also be employed to further enhance HNO binding as in the case of Cu−Ts discussed above. The reason that the electronic effect is not strong here is probably a result of the fact that HNO binding is weak, with ≈2.4–2.9 Å Cu−N distances.
After the HNO bound I-1 is formed, a redox reaction occurs to yield CuI and NO, see equation 1. In addition, HNO's proton is transferred to the nearby proton acceptor. In CuZnSOD, the proton accepting site is a negatively charged histidine ligand,12 which clearly has a stronger proton affinity than the neutral coordinating histidine ligands. Since the nearby nitrogen sites in Cu cyclams are all neutral, a systematic study of possible protonation sites in cyclams was performed. For CuDHX1, the reaction products with protons at each of the four coordinating nitrogens were optimized, which show that N1 with the electron-rich Bn substituent has the lowest energy and N3 which has the electron-donating Fl group is only higher by ≈3 kcal mol−1 and significantly more stable than the non-substituted N2/N4 sites by ≈10 kcal mol−1, Table S4. Therefore, the nitrogens with Bn and Fl groups are the preferred protonation sites respectively for CuDHX1 and Cu−Fl. For Cu−Ts, the nearby sulfonyl oxygen was also included in the protonation study. As seen from Table S5, N3 with the electron-withdrawing Ts substituent has the highest energy among all nitrogens (which are all better than the oxygen site to accept the proton), while its opposite site N1 has the most favorable protonation. Therefore, these preferred protonated species were used in the following reaction study. For Cu−Me4, the four nitrogens are symmetric.
Investigation of the TS connecting I-1 and I-2 (see Scheme 1 for a general reaction pathway with optimized molecular structures in Figure 1) shows that a proton-coupled electron transfer (PCET) mechanism operates here for all these Cu cyclams, analogous to HNO to NO conversion by the other metal complex11 and metalloproteins including both heme proteins13c and the non-heme protein.12 For example, in CuDHX1, there is a simultaneous electron transfer as indicated by the spin density results of NO (ραβNO) increasing from 0 e in I-1 to 0.1 e in TS then to ≈1.0 e in I-2, and accordingly Cu (ραβCu) decreasing from 0.47 e in I-1 to 0.46 e in TS then to ≈0 e in I-2, and the proton transfer as seen from the H−NO bond length elongation from 1.045 Å in I-1 to 1.059 Å in TS then to 2.082 Å in I-2, and the H−N1 distance shortening from 4.475 Å in I-1 to 1.897 Å in TS then to 1.027 Å in I-2. Namely, a hydrogen bond is formed between H and N1 at TS (this also occurs for other Cu cyclams, as indicated by 1.8–2.1 Å H⋅⋅⋅N1 distances in Table 2) to facilitate the proton transfer, which is absent in I-1 (RH⋅⋅⋅N1>4 Å, Table 2) since N1′s lone pair forms the coordination bond with Cu and thus is unavailable for hydrogen bonding. The proton transfer breaks the Cu−N1 coordination bond, see bond lengths of ≈2.1 Å in I-1 to ≈3.1 Å in TS and ≈3.2 Å in I-2 (Table 2), which makes N1 ready for the hydrogen bond with the incoming proton at TS. The qualitative reaction features of a barriered PCET and loss of one Cu coordination bond upon completion of proton transfer are identical to the first step of the native CuZnSOD reaction15 and the HNO to NO reactions mediated by CuZnSOD12 and its model.11 However, quantitatively the reaction barrier from I-1 to TS via CuZnSOD is lower than here by ≈9 kcal mol−1, which may be a result of 1) a more flexible non-planar coordination environment in the protein and thus a lower energy cost for breaking the Cu−N1 bond (≈1 Å elongation from I-1 to TS for Cu cyclams vs. only ≈0.4 Å elongation for CuZnSOD12) and 2) a stronger proton acceptor via the negatively charged coordinating histidine ligand in CuZnSOD than the neutral ligand here to reduce the proton transfer energy cost.
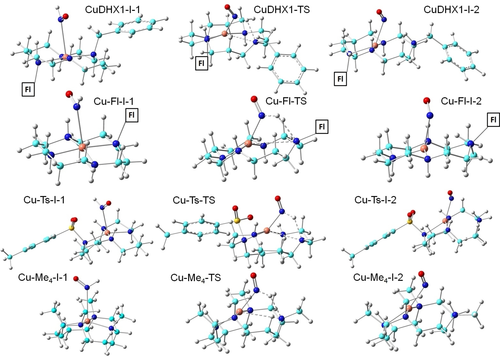
Optimized structures of species in the HNO to NO conversion step by Cu cyclams. Atom color scheme: C- cyan, N- blue, O- red, S- yellow, H- grey, Cu- orange. The Fl group is represented by its symbol for clarity with complete optimized structures in Figure S7. Dash line: bond in transition.
Apart from the above-mentioned geometric changes, the Cu−N distance undergoes a significant contraction (≈0.4–0.9 Å) for the forward barrier step I-1→TS, see Figure 2. The charge analysis reveals that a negative charge of ca. 0.07 e was transferred from HNO to Cu in this step, consistent with the electron transfer direction. The absolute charge transfer value trend of Cu−Me4<Cu−Ts ≈ CuDHX1<Cu−Fl is inversely correlated with the Gibbs free energies of activation (ΔG≠, I-1→TS) trend of Cu−Me4>Cu−Ts ≈ CuDHX1>Cu−Fl, although both ranges are small. To facilitate the comparison among the studied four Cu cyclams, all energies in this step shown in Table 2 and Figure 3 are with respect to the I-1′s reaction energy for CuDHX1, which was chosen because it is the only experimental system studied here with fluorescence based HNO detection and imaging.7g, 7k Cu−Me4 which bears the most sterically hindered substitutions has the largest ΔG≠ (see Table 2), while the other three cyclams with one or two substituents have similar barriers within 0.63 kcal mol−1 differences. This highlights the substituent's steric effect on the barrier.
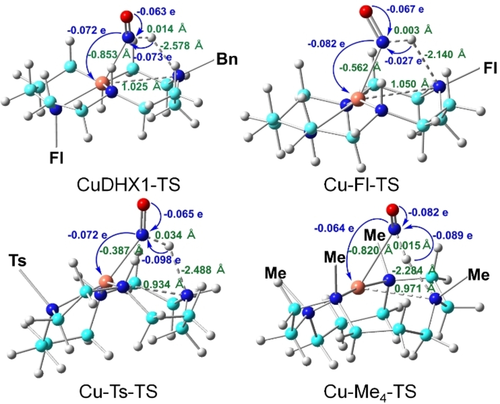
Atomic charge transfer for HNO (in blue) and key bond length changes (in green) from I-1 to TS for Cu cyclams drawn on the TS structures.
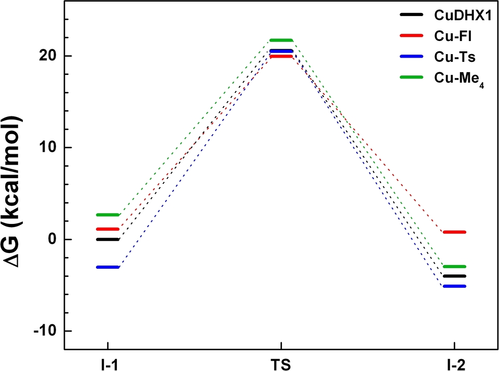
Gibbs free energies along the HNO to NO conversion pathways via Cu cyclams.
It should be noted that although it takes some energies to generate I-1, the experimental uses of 50 : 1 or even 100 : 1 ratio of HNO donor vs. Cu cyclam7g, 7k can significantly enhance the thermodynamic driving force for its formation especially in the cases of CuDHX1, Cu−Fl, and Cu−Ts, where a stable I-1 exists. This facilitates the accumulation of I-1 as the starting point for the HNO reaction and makes ΔG≠ of I-1→TS the effective overall barrier for such experimental reactions. Since their ΔG≠ values of ≈20 kcal mol−1 are close to some room temperature reactions,16 these kinetically feasible barriers support their experimentally observed reactivities. However, for Cu−Me4 which does not have a stable I-1 as found in the previous section, the high TS barrier from the starting reactant of 31.54 kcal mol−1 (see Table S10) accounts for its experimental inactivity toward HNO.
The reaction energies of this HNO to NO conversion step (I-1→I-2) are all negative (< −2 kcal mol−1) and thus thermodynamically favorable, except for Cu−Fl which is almost thermodynamically neutral (ΔG=−0.33 kcal mol−1). Cu−Fl is also the only Cu cyclam studied here to possess a positive enthalpy change for this reaction step, see Table 1. These results suggest that the HNO reaction with Cu−Fl is reversible or even a little unfavorable regarding ΔH. This feature has not been recognized before and does not favor the formation or accumulation of I-2 toward final NO releasing to complete the reaction. This helps understand the experimental results: 1) only a tiny fluorescent intensity change of Cu−Fl was observed after the addition of the same amount of HNO donors as with CuDHX1; 2) an EPR-silent CuI species was not detected,7g unlike the case of CuDHX1. The less favorable reaction energy of Cu−Fl vs. CuDHX1 by ≈4 kcal mol−1 higher (Table 1) is likely due to its relatively less favorable protonation energy by ≈3 kcal mol−1 in these two sites (Table S4) and its smaller redox potential by 0.045 V7g (≈1 kcal mol−1). This indicates that proton transfer is indeed a critical component of the PCET mechanism and a stronger EDG (such as Bn vs. Fl in these two systems) may help make the reaction thermodynamically more favorable.
Above results clearly show that both kinetic barriers and thermodynamic driving forces in the HNO to NO conversion step are important for the various experimental reactivities.
As seen from Table 1, reaction energies of NO releasing step (I-2→P+NO) for these Cu cyclams of −9 to −13 kcal mol−1 are all much more negative than those in other steps, indicating that this step provides the largest thermodynamic driving force for the overall reaction. This process is largely entropy-driven due to releasing NO gas, unlike the enthalpy-driven HNO to NO conversion step. These features are also similar to the HNO to NO reactions mediated by CuZnSOD12 and its model.11
Combining all steps, it is CuDHX1 (the only experimentally used HNO fluorescence senor out of these four Cu cyclams) that has the most negative net reaction Gibbs free energy (−7.38 kcal mol−1) and enthalpy (−6.60 kcal mol−1). The extra Bn substituent in CuDHX1 compared to Cu−Fl, although does not help bring down the barrier, plays a crucial role on making its reaction with HNO thermodynamically favorable. In contrast, the net reaction ΔG of −0.25 kcal mol−1 and ΔH of −0.12 kcal mol−1 for Cu−Fl, which are within computational errors to zeros, show that the net reaction is also basically reversible or not thermodynamically favorable, like the HNO to NO conversion step. This is consistent with its poor reactivity with HNO and makes Cu−Fl not a practical HNO senor. These results indicate the importance of a strong EDG on the proton acceptor to enhance the proton transfer needed for its PCET reactivity, which helps both the HNO to NO conversion and overall reaction.
Besides the electronic factor, the substituent's steric strain is another key influence on its HNO reactivity. In fact, the main reason Cu−Me4 with the same sufficiently high redox potential to oxidize HNO as Cu−Ts7k still exhibits no HNO reactivity is that the steric effect of its tetra-substitution abolishes a stable HNO binding intermediate and leads to a prohibitively high reaction barrier and relatively higher reaction energy, as discussed above. Namely, this steric effect reduces reactivity in every step. In contrast, Cu−Ts with an EWG (not a favorable electronic factor) still has facile HNO reactivity, because this system with just one substituent is of little steric hindrance and this EWG enhances the proton affinity of the proton acceptor site opposite to it. This shows that the substituent's steric effect is more important than its electronic nature.
Conclusion
In summary, this first study of substituent effect on metal complex based HNO sensors with various substitution situations reproduced the divergent experimental reactivities, although their reaction mechanisms are common as PCET. More importantly, a detailed theoretical understanding of previously unknown substituent effects was provided, which suggests the following guidelines to facilitate future HNO sensor development:
-
Steric effect is primary. The overcrowding substituents around the reaction center is not preferred. One or two substituents as in the cases of Cu−Ts and CuDHX1 may work.
-
Electronic effect is secondary: both EDG and EWG are good (if it has a sufficient redox potential to oxidize HNO), although the former is better to help the proton transfer. A combination of an EDG and an EWG on opposite sides of the coordination shell that has not been experimentally used before may also work, since this EWG may further enhance the EDG's role on increasing the proton affinity and thus reactivity as for Cu−Ts.
-
Substituent effect can be subtle (e.g. an unexpected poor HNO reactivity for Cu−Fl, which has only one substituent, least steric effect, and has a favorable EDG and redox potential). Therefore, a theoretical study for a designed case to examine the reaction pathway in detail can help ensure both a favorable kinetic barrier and a favorable thermodynamic reaction energy needed for an excellent HNO sensor before experimental realization, which will save time and cost.
In addition, the comprehensive HNO mechanistic results here may also help investigations of other HNO reactions with metal complexes.
Acknowledgements
This work was supported by an NIH grant GM085774 to YZ.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.