Cascade Cyclopolymerization of 5-Ethynyl-1,8-Nonadiyne Derivatives to Synthesize Low Band Gap Conjugated Polyacetylenes Containing a Fused Bicyclic Structure
Graphical Abstract
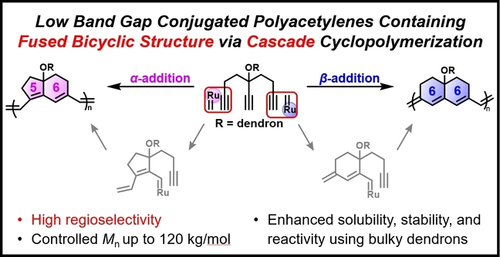
A novel cascade cyclopolymerization is developed to synthesize polyacetylenes containing fused bicyclic rings via sequential cascade ring-closing metathesis. The regioselectivity is controlled by altering the structure of the catalyst, affording polymers containing fused bicyclo[4,3,0] or [4,4,0] rings. The resulting polymers show narrow band gaps due to the planarization of the conjugated segment resulting from the fused bicyclic structure.
Abstract
Cyclopolymerization is a powerful method for synthesizing polyacetylenes containing four- to seven-membered rings. However, the structure of the repeat unit only consists of mono-cycloalkene due to the single cyclization of diyne monomers. Herein, we demonstrate a novel cascade cyclopolymerization to synthesize polyacetylenes containing fused bicyclic rings from triyne monomers containing bulky dendrons via sequential cascade ring-closing metathesis. These dendrons provided solubility and stability to the rigid bicyclic polyacetylene backbone. In addition, we controlled the regioselectivity of the catalyst approach by altering its structure and synthesized polymers containing fused bicyclo[4,3,0] or [4,4,0] rings with high molecular weights of up to 120 kg mol−1. Interestingly, the resulting polymers showed narrower band gaps (down to 1.6 eV) than polymers with mono-cycloalkene repeat units due to the planarization of the conjugated segment resulting from the fused bicyclic structure.
Conflict of interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available in the Supporting Information of this article.