Photoredox-Catalyst-Enabled para-Selective Trifluoromethylation of tert-Butyl Arylcarbamates
Yaqiqi Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorBao Li
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorNana Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorSai Shu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorYujie Chen
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorShan Yang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorZhibin Huang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Daqing Shi
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yingsheng Zhao
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorYaqiqi Jiang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorBao Li
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
These authors contributed equally to this work.
Search for more papers by this authorNana Ma
Collaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorSai Shu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorYujie Chen
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorShan Yang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorZhibin Huang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Daqing Shi
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Dr. Yingsheng Zhao
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123 P. R. China
School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, 453000 P. R. China
Search for more papers by this authorGraphical Abstract
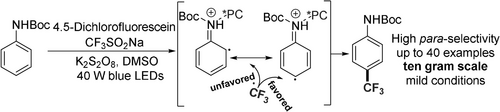
We report a light-promoted 4,5-dichlorofluorescein (DCFS)-enabled para-selective C−H trifluoromethylation of arylcarbamates using the Langlois reagent. Arylcarbamates, including the bioactive compound chlorzoxazone, vorinostat precursor, chlorpropham, and teriflunomide precursors, were suitable for this reaction, leading to the formation of the corresponding products in moderate to good yields.
Abstract
The direct incorporation of a trifluoromethyl group on an aromatic ring using a radical pathway has been extensively investigated. However, the direct highly para-selective C−H trifluoromethylation of a class of arenes has not been achieved. In this study, we report a light-promoted 4,5-dichlorofluorescein (DCFS)-enabled para-selective C−H trifluoromethylation of arylcarbamates using Langlois reagent. The preliminary mechanistic study revealed that the activated organic photocatalyst coordinated with the arylcarbamate led to para-selective C−H trifluoromethylation. Ten-gram scale reaction performs well highlighting the synthetic importance of this new protocol.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202105631-sup-0001-misc_information.pdf7.8 MB | Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788–802;
- 1bS. J. Lee, K. J. Makaravage, A. F. Brooks, P. J. H. Scott, M. S. Sanford, Angew. Chem. Int. Ed. 2019, 58, 3119–3122; Angew. Chem. 2019, 131, 3151–3154;
- 1cQ. Zheng, C. F. Liu, J. Chen, G. W. Rao, Adv. Synth. Catal. 2020, 362, 1406–1446.
- 2
- 2aM. T. Mihai, G. R. Genov, R. J. Phipps, Chem. Soc. Rev. 2018, 47, 149–171;
- 2bS. Porey, X. Zhang, S. Bhowmick, V. Kumar Singh, S. Guin, R. S. Paton, D. Maiti, J. Am. Chem. Soc. 2020, 142, 3762–3774;
- 2cG. Meng, N. Y. S. Lam, E. L. Lucas, T. G. Saint-Denis, P. Verma, N. Chekshin, J. Q. Yu, J. Am. Chem. Soc. 2020, 142, 10571–10591.
- 3
- 3aL. Yang, K. Semba, Y. Nakao, Angew. Chem. Int. Ed. 2017, 56, 4853–4857; Angew. Chem. 2017, 129, 4931–4935;
- 3bL. Yang, N. Uemura, Y. Nakao, J. Am. Chem. Soc. 2019, 141, 7972–7979.
- 4
- 4aR. Bisht, B. Chattopadhyay, J. Am. Chem. Soc. 2016, 138, 84–87;
- 4bM. E. Hoque, R. Bisht, C. Haldar, B. Chattopadhyay, J. Am. Chem. Soc. 2017, 139, 7745–7748.
- 5Y. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712–717.
- 6X. Lu, Y. Yoshigoe, H. Ida, M. Nishi, M. Kanai, Y. Kuninobu, ACS Catal. 2019, 9, 1705–1709.
- 7H. J. Davis, M. T. Mihai, R. J. Phipps, J. Am. Chem. Soc. 2016, 138, 12759–12762.
- 8
- 8aD. Leow, G. Li, T. S. Mei, J. Q. Yu, Nature 2012, 486, 518–522;
- 8bH. X. Dai, G. Li, X. G. Zhang, A. F. Stepan, J. Q. Yu, J. Am. Chem. Soc. 2013, 135, 7567–7571;
- 8cL. Wan, N. Dastbaravardeh, G. Li, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 18056–18059;
- 8dR.-Y. Tang, G. Li, J.-Q. Yu, Nature 2014, 507, 215–220;
- 8eG. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R. Y. Tang, M. Movassaghi, J. Q. Yu, J. Am. Chem. Soc. 2014, 136, 10807–10813;
- 8fJ. Xu, J. Chen, F. Gao, S. Xie, X. Xu, Z. Jin, J. Q. Yu, J. Am. Chem. Soc. 2019, 141, 1903–1907.
- 9
- 9aJ. Li, S. Warratz, D. Zell, S. De Sarkar, E. E. Ishikawa, L. Ackermann, J. Am. Chem. Soc. 2015, 137, 13894–13901;
- 9bZ. Fan, J. Ni, A. Zhang, J. Am. Chem. Soc. 2016, 138, 8470–8475;
- 9cJ. A. Leitch, C. G. Frost, Chem. Soc. Rev. 2017, 46, 7145–7153;
- 9dK. Jing, Z.-Y. Li, G.-W. Wang, ACS Catal. 2018, 8, 11875–11881;
- 9eK. Korvorapun, R. Kuniyil, L. Ackermann, ACS Catal. 2020, 10, 435–440.
- 10
- 10aS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 10bJ. Wang, M. Sanchez-Rosello, J. L. Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2014, 114, 2432–2506;
- 10cH. Ge, Q. Shen, Org. Chem. Front. 2019, 6, 2205–2209;
- 10dH. Ge, B. Wu, Y. Liu, H. Wang, Q. Shen, ACS Catal. 2020, 10, 12414–12424;
- 10eY. Ouyang, C.-L. Tong, X.-H. Xu, F.-L. Qing, Org. Lett. 2021, 23, 346–350.
- 11D. A. Nagib, D. W. MacMillan, Nature 2011, 480, 224–228.
- 12Y. Ouyang, X. H. Xu, F. L. Qing, Angew. Chem. Int. Ed. 2018, 57, 6926–6929; Angew. Chem. 2018, 130, 7042–7045.
- 13B. Yang, D. Yu, X.-H. Xu, F.-L. Qing, ACS Catal. 2018, 8, 2839–2843.
- 14Y. Zhang, T. S. Lee, J. M. Favale, D. C. Leary, J. L. Petersen, G. D. Scholes, F. N. Castellano, C. Milsmann, Nat. Chem. 2020, 12, 345–352.
- 15
- 15aY. Fujiwara, J. A. Dixon, F. O'Hara, E. D. Funder, D. D. Dixon, R. A. Rodriguez, R. D. Baxter, B. Herlé, N. Sach, M. R. Collins, Y. Ishihara, P. S. Baran, Nature 2012, 492, 95–99;
- 15bR. C. Simon, E. Busto, N. Richter, V. Resch, K. N. Houk, W. Kroutil, Nat. Commun. 2016, 7, 13323.
- 16K. Natte, R. V. Jagadeesh, L. He, J. Rabeah, J. Chen, C. Taeschler, S. Ellinger, F. Zaragoza, H. Neumann, A. Brückner, M. Beller, Angew. Chem. Int. Ed. 2016, 55, 2782–2786; Angew. Chem. 2016, 128, 2832–2836.
- 17W. Beatty, J. J. Douglas, R. Miller, R. C. McAtee, K. P. Cole, C. R. J. Stephenson, Chem 2016, 1, 456–472.
- 18
- 18aS. Cai, C. Chen, Z. Sun, C. Xi, Chem. Commun. 2013, 49, 4552–4554;
- 18bX. Gao, Y. Geng, S. Han, A. Liang, J. Li, D. Zou, Y. Wu, Y. Wu, Org. Lett. 2018, 20, 3732–3735;
- 18cE. Mejía, A. Togni, ACS Catal. 2012, 2, 521–527.
- 19R. K. Desiraju, N. L. Renzi, R. K. Nayak, K.-T. Ng, J. Pharm. Sci. 1983, 72, 991–994.
- 20
- 20aE. Weisberg, P. Manley, J. Mestan, S. Cowan-Jacob, A. Ray, J. D. Griffin, Br. J. Cancer 2006, 94, 1765–1769;
- 20bBeena, D. S. Rawat, Med. Res. Rev. 2013, 33, 693–764.
- 21
- 21aL.-S. Zhang, K. Chen, G. Chen, B.-J. Li, S. Luo, Q.-Y. Guo, J.-B. Wei, Z.-J. Shi, Org. Lett. 2013, 15, 10–13;
- 21bJ. Xu, L. Qiao, J. Shen, K. Chai, C. Shen, P. Zhang, Org. Lett. 2017, 19, 5661–5664;
- 21cC. Xia, K. Wang, G. Wang, G. Duan, Org. Biomol. Chem. 2018, 16, 2214–2218;
- 21dL. Zou, P. Li, B. Wang, L. Wang, Chem. Commun. 2019, 55, 3737–3740;
- 21eC. Tian, Q. Wang, X. Wang, G. An, G. Li, J. Org. Chem. 2019, 84, 14241–14247.
- 22
- 22aC. Tian, X. Yao, W. Ji, Q. Wang, G. An, G. Li, Eur. J. Org. Chem. 2018, 5972–5979;
- 22bC. Yuan, P. Dai, X. Bao, Y. Zhao, Org. Lett. 2019, 21, 6481–6484.
- 23F. Ye, F. Berger, H. Jia, J. Ford, A. Wortman, J. Borgel, C. Genicot, T. Ritter, Angew. Chem. Int. Ed. 2019, 58, 14615–14619; Angew. Chem. 2019, 131, 14757–14761.
- 24
- 24aW. T. Fan, Y. Li, D. Wang, S. J. Ji, Y. Zhao, J. Am. Chem. Soc. 2020, 142, 20524–20530;
- 24bG. Tu, C. Yuan, Y. Li, J. Zhang, Y. Zhao, Angew. Chem. Int. Ed. 2018, 57, 15597–15601; Angew. Chem. 2018, 130, 15823–15827.
- 25A. B. Viveki, D. N. Garad, R. G. Gonnade, S. B. Mhaske, Chem. Commun. 2020, 56, 1565–1568.
- 26
- 26aH. Huo, K. Harms, E. Meggers, J. Am. Chem. Soc. 2016, 138, 6936–6939;
- 26bZ. H. Luan, J. P. Qu, Y. B. Kang, J. Am. Chem. Soc. 2020, 142, 20942–20947;
- 26cH. L. Zhu, F. L. Zeng, X. L. Chen, K. Sun, H. C. Li, X. Y. Yuan, L. B. Qu, B. Yu, Org. Lett. 2021, 23, 2976–2980.