GlycoBODIPYs: Sugars Serving as a Natural Stock for Water-soluble Fluorescent Probes of Complex Chiral Morphology
Dedicated to Professor Horst Kunz on the occasion of his 80th birthday
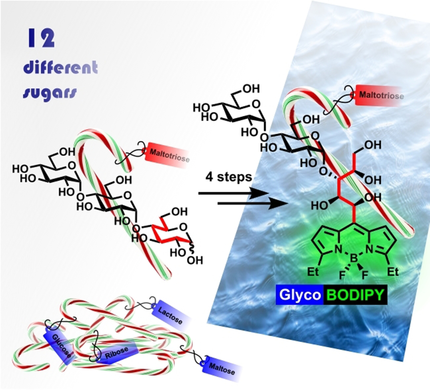
Graphical Abstract
Twelve sugars, differing in size and stereochemistry, were used as building blocks in a modular, efficient four-step synthetic route to multifunctional BODIPY glycoconjugates with high fluorescence and hydrophilicity. The chiral information was transferred unchanged, thereby providing complex and distinct probes for target-oriented binding studies and specific in vivo imaging.
Abstract
A range of unprocessed, reducing sugar substrates (mono-, di-, and trisaccharides) is shown to take part in a straightforward four-step synthetic route to water-soluble, uncharged BODIPY derivatives with unimpaired chiral integrity and high fluorescence efficiency. A wide compatibility with several postfunctionalizations is demonstrated, thus suggesting a universal utility of the multifunctional glycoconjugates, which we call GlycoBODIPYs. Knoevenagel condensations are able to promote a red-shift in the spectra, thereby furnishing strongly fluorescent red and far-red glycoconjugates of high hydrophilicity. The synthetic outcome was studied by X-ray crystallography and by comprehensive photophysical investigations in several solvent systems. Furthermore, cell experiments illustrate efficient cell uptake and demonstrate differential cell targeting as a function of the integrated chiral information.
Even though sometimes denounced as “porphyrin's little sister”, the compact BODIPY scaffold has made triumphal progress in all facets of life science since its discovery in the 1960s.1 As a true member of the cyanine family of dyes, it contains distinctive nitrogen capping units that are integrated into two planar pyrrole moieties, which provide strong anchor points for a central, chelated BF2 group, thus promoting both skeletal stiffness and fluorescence properties. In conjunction with a wide range of postfunctionalizations,2 the typical absorption of the BODIPY motif at ca. 500 nm can be modified either by extension of the π-system3 or by electronic stabilization at its crucial meso position4 (aza-BODIPYs,5 (aza-)BOIMPYs6 etc.). However, in contrast to the specific structural nature of cyanines (rhodamines), which bear a positive charge in their π-systems, the BODIPY platform is inherently uncharged and thus of very restricted hydrophilicity. Additionally, a high planarity and C2v symmetry tend to trigger aggregation processes, either of the single fluorophore itself4, 7 or of labeled (bio-)molecules.8 This aggregation can impose severe limitations on conjugation processes,9 the image quality in modern (super-resolution) microscopy techniques,10 and general applications in aqueous media (sensors, photosensitizers etc.).11 Carbohydrates, on the other hand, encompass a diverse set of modularly assembled biomolecules of the highest structural and chiral complexity. Although the number of hydroxy groups usually provides reasonable water-solubility, their specific pattern and arrangement are crucial for nature's unique lock and key approach, which is responsible for numerous recognition and signaling processes throughout the living world.12 The determination of human blood types, for example, is based on the exposure of distinct glycan residues on blood cell surfaces. In addition, toxins, such as those from Vibrio cholerae or Shigella dysenteriae, can also trigger severe diseases of the digestive tract by exploiting certain carbohydrate signatures (GM113 and Gb314) of the host for intrusion and pathogenicity. However, the entire structural identity of a carbohydrate moiety is not usually necessary for the recognition process to take place, as the work of Feizi et al. seminally proved.15 Certain hydroxy functions or even complete sugar units can be redundant or derivatized,16 as was also recently found in our group.17 Although BODIPYs have already been transformed into water-soluble analogues by various approaches (Scheme 1) such as by sulfonation,18 introduction of ammonium,19 carboxylate,20 multiple PEG residues,21 or recently by a bistriflyl-substituted carbanion,22 most of them suffer from a tedious synthetic route (sulfonated peptidyl linkers),19 the incorporation of unnecessary structural ballast (PEG), or from the introduced charge, which can impair facile cell-uptake and further functionalizations in organic media. Furthermore, the native fluorescence of BODIPYs can often be substantially compromised after the derivatization process (PEG, peptidyl linkers, meso pyridinium23).
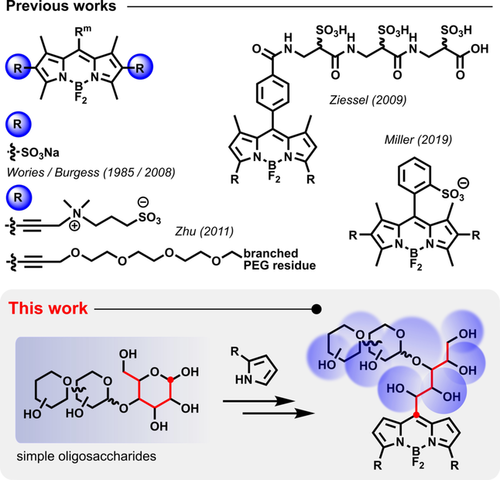
Previous studies on water-soluble BODIPY scaffolds and our approach, which involves naturally abundant sugar substrates.
Herein we present a straightforward and versatile synthetic route to convert a wide range of naturally abundant, reducing sugar species into highly fluorescent and water-soluble BODIPY derivatives. Our strategy exploits the anomeric center as a reactive site for an initial pyrrole condensation, which opens up the cyclic structure of the involved sugar moiety, while not compromising the inherent chiral information. Although monosaccharides thereby sacrifice their cyclic shape, di- and trisaccharides preserve the structural identity of their additional ring structures, which renders them especially useful for biomolecular recognition assays.
To engage the hemiacetal function of carbohydrates in a common pyrrole condensation, we took advantage of the Lewis acids Sc(OTf)3 and Yb(OTf)3 in a carefully degassed H2O/EtOH solvent system at elevated temperatures (Scheme 2). Unprecedentedly, and in contrast to former reports with indoles,24 even fully unprotected sugars smoothly gave corresponding C-glycosides 2sugar in up to quantitative yields when reacted with a slight excess of an α-monosubstituted pyrrole reaction partner. We decided to use ethylpyrrole as the most easily available and economic starting material and also expected an improved shielding of the sensitive and hydrolyzable BF2 unit. However, methylpyrrole proved to be fully comparable in all reaction steps in terms of yield, purification, and, to our surprise, also stability, which was proved exemplarily with d-lactose 1Lac as substrate (see the Supporting Information). Initial trials to directly use the generated glyco-dipyrromethanes for the formation of a BODIPY by using adjusted synthetic procedures were unsuccessful, a result perhaps to be expected considering the number of hydroxy groups and the strong oxophilicity of boron trifluoride. Fortunately, we were able to circumvent this deadlock with an efficient protection-deprotection strategy. Applying specific conditions, even 11 hydroxy groups of the largest, maltotriose-derived, C-glycoside 2Mal3 were easily protected (using the triethylsilyl (TES) group) with a TESOTf/1-methylpyridine system. Thanks to the imposed hydrophobicity, the reaction mixture could often be simply purified by a quick filtration step over deactivated silica gel without further chromatographic efforts. The TES-protected dipyrromethanes 3sugar thus isolated proved to be well-suited substrates to enter a standard BODIPY synthesis and furnished protected GlycoBODIPYs in 30–50 % yield. However, the reaction time and the ratio of NEt3 and BF3⋅OEt2 need to be handled with care, since a slow, insidious cleavage of the TES group is likely to happen otherwise. The synthetic route ends with a one-pot deprotection procedure with catalytically active HCl used as a cheap proton source. The reaction appears quantitative by thin-layer chromatography, but its efficiency is mainly determined by the subsequent purification step. Even though HPLC might be regarded as the method of choice, we wished in general to avoid cost-intensive techniques to ensure the wide availability of our synthetic approach. Indeed, the whole range of final GlycoBODIPYs was attained with sufficient purity either by chromatography on silica gel with MeOH/EtOAc and/or by precipitation from a THF/Et2O solvent system.
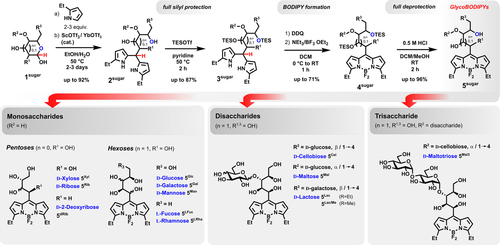
Synthetic strategy to a range of GlycoBODIPYs.
As anticipated, the water-solubility of the product series strongly depends on the number of integrated hydroxy groups. Whereas pentose-derived species have very limited solubility in water, GlycoBODIPYs derived from di- and trisaccharide substrates approach the solubility characteristics of the initial glycan. Furthermore, we found all of the final products to be soluble in THF, which provides compatibility for a range of optional postfunctionalizations. To challenge the synthetic versatility, we submitted the final glycoconjugates and intermediates to a selection of derivatization modes. Methylpyrrole-derived BODIPYs are known to condense with arylcarbaldehydes under Knoevenagel conditions3a to give the corresponding strong, red-shifted emitters. The TES-protected species 3Lac/Me underwent a smooth conversion into styryl-equipped congeners in refluxing benzene, without signs of detrimental deprotection processes, when the pH was kept in the mildly basic range (Scheme 3, top). Depending on the reaction time, either the monofunctionalized product 6Lac/Me or the di-substituted BODIPY 7Lac/Me could be isolated in high yields, usually together with starting material. Applying the standard deprotection procedure finally furnished the GlycoBODIPYs 8Lac/Me and 9Lac/Me with comparable yields. In addition to the chiral topology, the large number of hydroxy groups offers a unique opportunity for a general multifunctionalization of a dipyrrin platform. We thus tested exemplarily if a full benzoylation might be achievable. Indeed, all the hydroxy groups of 4Lac/Me were derivatized, even the obstructed group adjacent to the π-system, which however was found to be prone to undergo elimination if the temperature was further elevated (Scheme 3, middle). A selective diiodination of the pyrrolic β-positions is also transferable to the glycoconjugate, thereby providing a nonfluorescent dye scaffold for efficient singlet oxygen production in proximity to the glycan receptor unit.25
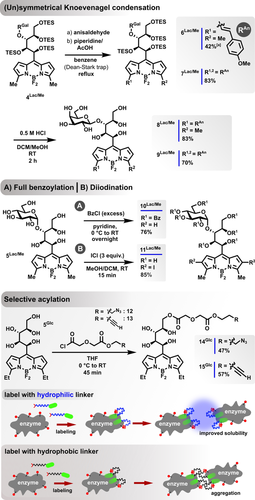
Investigated postfunctionalizations of GlycoBODIPYs and their precursors. [a] 40 % of the starting material was recovered.
Despite the chiral complexity, the integrated glycan moiety might also be regarded as a simple, highly hydrophilic, yet uncharged tether unit capped by a terminal, primary alcohol group. This molecular setup addresses a frequently encountered obstacle in common biolabeling procedures, in which the imposed hydrophobicity of the fluorescent tag, usually attached as a linker–emitter conjugate, often induces adverse precipitation of the charged biomolecule in aqueous media.8 This leads to low or arbitrary density of labeling (DOL) values and severe purification problems (Scheme 3, bottom).9 Very recently, the group of Hell was able to overcome this effect, inter alia, with the help of a separately employed, short glyco-azide linker, which proved the beneficial impact even of small carbohydrate units.10 On the basis of these results, we thus envisioned the transformation of a GlycoBODIPY into a fluorescent tag, which counterbalances the problematic hydrophobic nature of the BODIPY motif by an exposed hydrophilic linking unit. Employing the azide- and alkynyl-substituted acid chlorides 12 and 13, a selective acylation of the primary alcohol function of the glucose-derived GlycoBODIPY 5Glc was achieved by careful adjustment of the reaction time and stoichiometry. Products 14Glc and 15Glc still maintain sufficient water-solubility and offer a direct engagement in ligations through common click chemistry.
All of our glycoconjugates exhibit excellent absorption and emission qualities, which virtually coincide with the prototypical values known for the BODIPY scaffold. Quantum yields of fluorescence reach values close to 90 % in H2O and even PBS buffer solutions, and are thus effectively independent of the solvent polarity. Figure 1 exemplarily highlights some fundamental photophysics and traces the spectral impact of the Knoevenagel condensation with lactose-derived GlycoBODIPY 5Lac/Me in H2O and MeOH. Although the type of the attached carbohydrate scarcely influences the main absorption band of final products 5, one or two electron-donating styryl groups promote a red-shift towards 574 nm or 647 nm, respectively. BODIPYs are also known to undergo a red-shift of their main absorption with an electronically depleted meso position. This tendency is reflected in the series of our GlycoBODIPYs 5, but in a delicate manner. The deoxyribose-derived conjugate 5dRib, bearing an unsubstituted methylene unit adjacent to the meso position, does not perceive the electron-withdrawing strength of the hydroxy function and thus exhibits a slightly blue-shifted absorption at 504 nm in MeOH. More interesting, however, is the glucose-derived species 5Glc. As an epimer of 5Gal, it shows a significantly red-shifted main absorption at 514 nm (MeOH) compared to 5Gal (507 nm/MeOH), especially in solvent systems of lower polarity (see Figure 1, insert). The flipped stereocenter, however, is three bonds away from the crucial π- system and, alone, should not be able to influence the electronic constitution. Since the effect is more pronounced in MeOH and THF than in water/PBS, we assume that a conformational change in the carbohydrate moiety triggers a cooperative pull effect from all the hydroxy groups, thereby leading to an increased electron deficiency at the regulating meso position. To the best of our knowledge such an impact of remote point chirality on excitation energies is unexpectedly high and unprecedented. To scrutinize the configurational integrity of the carbohydrate moieties we invested much effort in the crystallization of our final products.
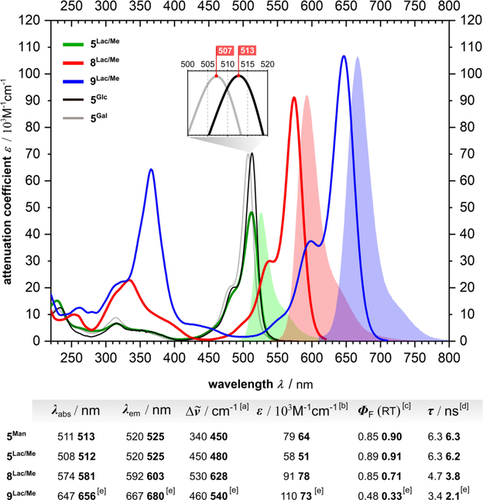
Absorption and normalized emission spectra of 5Lac/Me (H2O) and its styryl-equipped congeners 8Lac/Me and 9Lac/Me in MeOH at RT together with a short overview of spectroscopic data recorded in MeOH and H2O (bold). Lines: absorptions, filled areas: corresponding emissions. [a] Stokes shift. [b] Attenuation coefficient at the main absorption band λabs. [c] Absolute quantum yield of fluorescence. [d] Fluorescence lifetime. Insert: Juxtaposition of the absorption spectra of 5Glc and 5Gal (MeOH). [e] 20 % MeOH was used as cosolvent because of an incipient aggregation process. See the Supporting Information for the full data set.
We eventually succeeded in obtaining crystals of the l-fucose-derived species 5LFuc (Figure 2). All stereocenters were found to correspond with the single configurations in the starting materiaL l-fucose, strongly indicating an unchanged chirality in the entire synthetic process (an unambiguous signal set in all 13C NMR spectra further supports the stereoisomeric purity, see the Supporting Information). The carbon atoms of the carbohydrate backbone consistently adopt antiperiplanar conformations with negligible global distortion. This results in a gauche-anti-gauche sequence regarding the orientations of the hydroxy groups starting from C5. No significant intramolecular hydrogen bonds are present; instead, all adjacent OH groups point in opposite directions, thereby enabling a crystal packing assisted by intermolecular hydrogen bonding (see the Supporting Information). Interestingly, the dihedral angle between the plane of the π-system and the elongated bond C14−C15 is very close to 90°, thus suggesting a hyperconjugation effect with the large orbital coefficient of the LUMO at carbon atom C5.
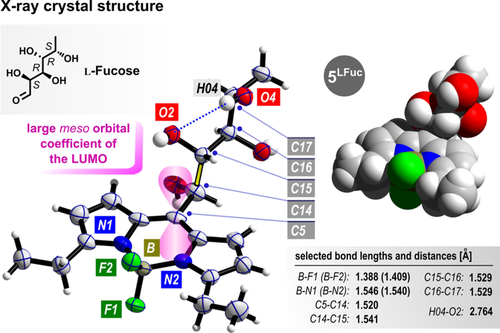
Molecular structure of 5LFuc and selected bond lengths and distances obtained by X-ray diffraction analysis. The synthetic formula is shown for comparison and to illustrate the position of stereocenters.
We then used confocal microscopy to demonstrate the advanced applicability of GlycoBODIPYs in live cell imaging experiments. We found facile cellular uptake of GlycoBODIPYs 5Sugar from the extracellular medium after an incubation period of 1 h (c=7.5 μm; Figure 3). The increased solubility of our dyes compared to a native BODIPY scaffold becomes evident as we observed aggregation of the latter under these conditions (Figure 3 C, see also Figures S138 and S139). Remarkably, a distinct subcellular enrichment as a function of the integrated chirality was detected. The most striking difference was found between the diastereomers 5LFuc and 5LRha. Whereas 5LRha is excluded from the nucleus, 5LFuc is located in the cytoplasm as well as in the nucleus and efficiently stains nucleoli (Figures 3 A,B and S140). This comparison demonstrates the functionality of the integrated chiral information in the cellular context and its activity within a synthetically reshaped glycan unit. To further analyze the staining pattern and to identify the targeted subcellular components, we submitted all GlycoBODIPYs to co-localization studies with organelle-specific fluorescent markers. Although no co-localization with a lysotracker was evident in any case, the d-cellobiose-derived GlycoBODIPY 5Cel exhibits high specificity for mitochondrial staining (Figure 3 D–F) in contrast to its epimeric congener 5Lac (see the Supporting Information for details).
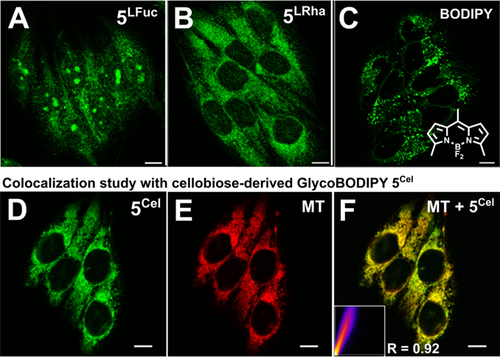
Confocal microscopy images of HeLa cells incubated with A) l-fucose-derived GlycoBODIPYs 5LFuc and B) and l-rhamnose-derived 5LRha. C) Corresponding staining result with usual BODIPY species lacking a glycan moiety. D–F) Colocalization study with cellobiose-derived GlycoBODIPY 5Cel (D) and MitoTracker™ Deep Red FM (MT) (E). F) Merged images; insert: correlation plot (heatmap) for visualization of the colocalization quality, R is the Pearson correlation coefficient (see the Supporting Information for the full data set).
In conclusion, we have proved that the chiral complexity of naturally abundant glycan substrates can be directly integrated into a BODIPY conjugate, thus opening up a modular route to tailor-made, water-soluble, and highly fluorescent probes that can meet the challenges of modern, target-specific recognition assays. In addition to the range of substrates presented here, specifically prepared and/or complex, hemiacetal-equipped glycans with a pure hydroxyl functionality, such as Gb3, also represent compatible and integratable oligosaccharides. A sequential red-shift in the absorption was realized by a single additional synthetic step, thus facilitating compatibility with multicolor labeling studies. Owing to the broad solubility range, several structural modifications can be realized within the synthetic route and these are able to transform the glycoconjugates into uncharged, hydrophilic labels for click chemistry mediated ligations and probes for target-specific photodynamic therapy. The spectroscopic advantages of the BODIPY motif are completely preserved in the assembled glycoconjugates, which show a widely solvent-independent fluorescence efficiency close to 90 %, irrespective of the integrated sugar moiety. Confocal microscopy experiments demonstrated a diverse applicability of GlycoBODIPYs for cellular studies and illustrate the potent chiral activity for specific cellular targets.
Acknowledgements
L.J.P. and S.A. acknowledge the Alexander von Humboldt Foundation for their postdoctoral fellowships. O.L. and S.E. acknowledge funding from the Research Training Group “Protein Complex Assembly” (DFG-GRK 2223). Open access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no conflict of interest.