Unprecedented Reactivity of γ-Amino Cyclopentenone Enables Diversity-Oriented Access to Functionalized Indoles and Indole-Annulated Ring Structures
Chenna Jagadeesh
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorBiplab Mondal
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorSourav Pramanik
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorProf. Dr. Dinabandhu Das
School of Physical Sciences, Jawaharlal Nehru University, New Delhi, India
Search for more papers by this authorCorresponding Author
Prof. Dr. Jaideep Saha
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorChenna Jagadeesh
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorBiplab Mondal
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorSourav Pramanik
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorProf. Dr. Dinabandhu Das
School of Physical Sciences, Jawaharlal Nehru University, New Delhi, India
Search for more papers by this authorCorresponding Author
Prof. Dr. Jaideep Saha
Division of Molecular Synthesis & Drug Discovery, Centre of Biomedical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 Uttar Pradesh, India
Search for more papers by this authorGraphical Abstract
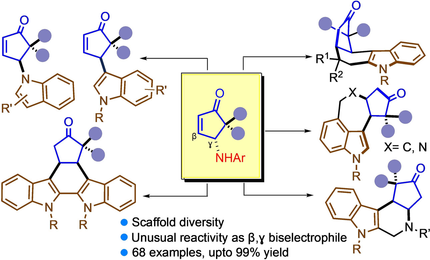
This work highlights diversity-oriented access to cyclopentenoid indoles and indole-alkaloid like structures which is pillared on an unprecedented latent reactivity of γ-aminoenones. The transformation highlights γ- or β,γ-functionalization of enones to generate new structural space. A reverse-aza-Piancatelli rearrangement seemed to be operating, which should invoke new attributes to its mechanism.
Abstract
Observation of an unexpected, Lewis acid promoted displacement of latent reactive γ-amino group on cyclopentenone presented unparalleled opportunity for enone functionalization and annulations with indole derivatives, which is developed in the current study. Herein, a vast range of C3/N-indolyl enones and indole alkaloid-like compound were accessed in excellent yields (up to 99 %) and selectivity through a one-pot operation. The mechanism most likely involves an unprecedented trait of Piancatelli-type rearrangement where influence of the gem-diaryl group appeared crucial.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202016015-sup-0001-misc_information.pdf18.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aD. J. Aitken, H. Eijsberg, A. Frongia, J. Ollivier, P. P. Piras, Synthesis 2014, 46, 1–24;
- 1bS. P. Simeonov, J. P. M. Nunes, K. Guerra, V. B. Kurteva, C. A. M. Afonso, Chem. Rev. 2016, 116, 5744–5893.
- 2
- 2aG. Piancatelli, A. Scettri, S. Barbadoro, Tetrahedron Lett. 1976, 17, 3555–3558;
10.1016/S0040-4039(00)71357-8 Google Scholar
- 2bC. Verrier, S. Moebs-Sanchez, Y. Queneau, F. Popowycz, Org. Biomol. Chem. 2018, 16, 676–687.
- 3For a selected review on Nazarov reactions, see:
- 3aM. J. Riveira, L. A. Marsili, M. P. Mischne, Org. Biomol. Chem. 2017, 15, 9255;
- 3bA. V. Yadykov, V. Z. Shirinian, Adv. Synth. Catal. 2020, 362, 702, and references therein; For examples relevant to current study, see:
- 3cR. William, S. Wang, F. Ding, E. N. Arviana, X.-W. Liu, Angew. Chem. Int. Ed. 2014, 53, 10742; Angew. Chem. 2014, 126, 10918;
- 3dA.-S. Marques, T. Duhail, J. Marrot, I. Chataigner, V. Coeffard, G. Vincent, X. Moreau, Angew. Chem. Int. Ed. 2019, 58, 9969; Angew. Chem. 2019, 131, 10074.
- 4For examples of Aza-Piancatelli reaction, see:
- 4aS.-W. Li, R. A. Batey, Chem. Commun. 2007, 3759–3761;
- 4bG. K. Veits, D. R. Wenz, J. Read de Alaniz, Angew. Chem. Int. Ed. 2010, 49, 9484–9487; Angew. Chem. 2010, 122, 9674–9677;
- 4cL. I. Palmer, J. Read de Alaniz, Angew. Chem. Int. Ed. 2011, 50, 7167–7170; Angew. Chem. 2011, 123, 7305–7308;
- 4dD. Lebœuf, E. Schulz, V. Gandon, Org. Lett. 2014, 16, 6464–6467;
- 4eD. Yu, V. T. Thai, L. I. Palmer, G. K. Veits, J. E. Cook, J. Read de Alaniz, J. E. Hein, J. Org. Chem. 2013, 78, 12784–12789;
- 4fY. Cai, Y. Tang, I. Atodiresei, M. Rueping, Angew. Chem. Int. Ed. 2016, 55, 14126–14130; Angew. Chem. 2016, 128, 14332–14336.
- 5For examples of domino aza-Piancatelli transformations, see:
- 5aB. V. Subba Reddy, Y. V. Reddy, K. Singarapu, Org. Biomol. Chem. 2016, 14, 1111;
- 5bK. Nayani, R. Cinsani, A. Hussaini SD, P. S. Mainkar, S. Chandrasekhar, Eur. J. Org. Chem. 2017, 5671;
- 5cL. Marin, V. Gandon, E. Schulz, D. Lebœuf, Adv. Synth. Catal. 2017, 359, 1157–1163;
- 5dB. Baldé, G. Force, L. Marin, R. Guillot, E. Schulz, V. Gandon, D. Leboeuf, Org. Lett. 2018, 20, 7405;
- 5eZ. Wei, J. Zhang, H. Yang, G. Jiang, Org. Lett. 2019, 21, 2790;
- 5fS. Gouse, N. R. Reddy, S. Baskaran, Org. Lett. 2019, 21, 3822;
- 5gS. Wang, R. Guillot, J.-F. Carpentier, Y. Sarazin, C. Bour, V. Gandon, D. Leboeuf, Angew. Chem. Int. Ed. 2020, 59, 1134–1138; Angew. Chem. 2020, 132, 1150–1154.
- 6Examples on carbo-Piancatelli rearrangement:
- 6aB. Yin, L. Huang, X. Wang, J. Liu, H. Jiang, Adv. Synth. Catal. 2013, 355, 370–376;
- 6bJ. Xu, Y. Luo, H. Xu, Z. Chen, M. Miao, H. Ren, J. Org. Chem. 2017, 82, 3561.
- 7
- 7aJ. M. Winne, S. Catak, M. Waroquier, V. Van Speybroeck, Angew. Chem. Int. Ed. 2011, 50, 11990; Angew. Chem. 2011, 123, 12196;
- 7bJ. Guo, B. Yu, Y.-N. Wang, D. Duan, L.-L. Ren, Z. Gao, J. Gou, Org. Lett. 2014, 16, 5088.
- 8For selected examples, see:
- 8aQ.-L. Xu, L.-X. Dai, S.-L. You, Chem. Sci. 2013, 4, 97;
- 8bL. Huang, L.-X. Dai, S.-L. You, J. Am. Chem. Soc. 2016, 138, 5793;
- 8cJ. B. Hendrickson, J. Wang, Org. Lett. 2004, 6, 3;
- 8dE. Fillion, A. M. Dumas, J. Org. Chem. 2008, 73, 2920;
- 8eD.-J. Cheng, H.-B. Wu, S.-K. Tian, Org. Lett. 2011, 13, 5636;
- 8fH. Schönherr, J. L. Leighton, Org. Lett. 2012, 14, 2610;
- 8gH. J. Lim, J. C. Gallucci, T. V. RajanBabu, Org. Lett. 2010, 12, 2162;
- 8hZ. Qiao, Z. Shafiq, L. Liu, Z.-B. Yu, Q.-Y. Zheng, D. Wang, Y.-J. Chen, Angew. Chem. Int. Ed. 2010, 49, 7294; Angew. Chem. 2010, 122, 7452;
- 8iS. L. Crawley, R. L. Funk, Org. Lett. 2003, 5, 3169;
- 8jC. Cordier, D. Morton, S. Murrison, A. Nelson, C. O'Leary-Steele, Nat. Prod. Rep. 2008, 25, 719.
- 9
- 9aI. Komoto, S. Kobayashi, Org. Lett. 2002, 4, 1115–1118;
- 9bG. Bartoli, M. Bartolacci, M. Bosco, G. Fogila, A. Giuliani, E. Marcantoni, L. Sambri, E. Torregiani, J. Org. Chem. 2003, 68, 4594;
- 9cW. Liu, S. Rajkumar, W. Wu, Z. Huang, X. Yang, Org. Lett. 2019, 21, 3563, and references therein.
- 10Michael addition is reported in presence of aniline/amines. For example, see:
- 10aW. Li, T. Werner, Org. Lett. 2017, 19, 2568;
- 10bS. Kobayashi, K. Kakumoto, M. Sugiura, Org. Lett. 2002, 4, 1319.
- 11γ-Functionalization of enone has emerged as an attractive strategy. For relevant examples, see:
- 11aN. Di Iorio, P. Righi, A. Mazzanti, M. Mancinelli, A. Ciogli, G. Bencivenni, J. Am. Chem. Soc. 2014, 136, 10250–10253;
- 11bC. Zou, C. Zeng, Z. Liu, M. Lu, X. Sun, J. Ye, Angew. Chem. Int. Ed. 2016, 55, 14257–14261; Angew. Chem. 2016, 128, 14469–14473.
- 12Retro-Nazarov reaction is known. See: M. Harmata, D. R. Lee, J. Am. Chem. Soc. 2002, 124, 14328.
- 13Furan-2-yldiphenylmethanol (1′a) showed similar efficiency with 1H-indole (92 %) under this condition.
- 14Multiple spot formation was observed in TLC analysis.
- 15For selected examples of (indolyl) N-C chiral axis, see:
- 15aK. Kamikawa, S. Kinoshita, M. Furusyo, S. Takemoto, H. Matsuzaka, M. Uemura, J. Org. Chem. 2007, 72, 3394;
- 15bG. Bringmann, S. Tasler, H. Endress, J. Kraus, K. Messer, M. Wohlfarth, W. Lobin, J. Am. Chem. Soc. 2001, 123, 2703–2711;
- 15cZ. Li, M. Tang, C. Hu, S. Yu, Org. Lett. 2019, 21, 8819–8823.
- 16
- 16aZ. Wang, H. Zeng, C.-J. Li, Org. Lett. 2019, 21, 2302–2306;
- 16bB.-J. Peng, W.-T. Hsueh, F. Fulop, S.-C. Yang, New J. Chem. 2019, 43, 58.
- 17T. Janosik, A. Rannug, U. Rannug, N. Wahlstorm, J. Slatt, J. Bergman, Chem. Rev. 2018, 118, 9058–9128.
- 18Intractable product mixture were obtained in these cases. For gem-diethyl substituted 2-furfurylcarbinol, starting materials were decomposed.
- 19F. Tan, H.-G. Cheng, Chem. Commun. 2019, 55, 6151–6164, and references therein.
- 20Selective reactions were performed with furan-2-yldiarylmethanol (Ph, p-OMe, m-OMe etc.) under standard conditions. Intractable product mixture was obtained for these cases.
- 21
- 21aE. D. Cox, J. M. Cook, Chem. Rev. 1995, 95, 1797;
- 21bA. E. Laine, C. Lood, A. M. P. Koskinen, Molecules 2014, 19, 1544–1567;
- 21cM. Bandini, A. Mellono, F. Piccinelli, R. Sinisi, S. Tommasi, A. Umani-Ronchi, J. Am. Chem. Soc. 2006, 128, 1424;
- 21dE. D. Cox, H. Diaz-Harauzo, Q. Huang, M. S. Reddy, C. Ma, B. Harris, R. McKernan, P. Skolnick, J. M. Cook, J. Med. Chem. 1998, 41, 2537.
- 22Reaction of 2-furyl carbinol and indolyl sulfonamide was futile.
- 23
- 23aJ. A. MacKay, R. L. Bishop, V. H. Rawal, Org. Lett. 2005, 7, 3421;
- 23bR. W. Heidebrecht, B. Gulledge, S. F. Martin, Org. Lett. 2010, 12, 2492;
- 23cX. Tian, A. D. Huters, C. J. Douglas, N. K. Garg, Org. Lett. 2009, 11, 2349;
- 23dK. S. Feldman, P. Ngernmeesri, Org. Lett. 2011, 13, 5704;
- 23eB. Q. Li, Z.-W. Qiu, A.-J. Ma, J.-B. Peng, N. Feng, J.-Y. Du, H.-P. Pan, X.-Z. Zhang, X.-T. Xu, Org. Lett. 2020, 22, 1903.
- 24
- 24aA. C. Lindsay, S. H. Kim, J. Sperry, Nat. Prod. Rep. 2018, 35, 1347–1382;
- 24bH. Liu, Y. Jia, Nat. Prod. Rep. 2017, 34, 411–432.
- 25Use of BCl3 was not compatible with indoles having free -NH, and reaction time was much longer in comparison to that of BF3. For a relevant example citing differences in Lewis acidity of BF3 and BCl3, see: F. Bessac, G. Frenking, Inorg. Chem. 2003, 42, 7990.
- 26M. Braun, Angew. Chem. Int. Ed. 2012, 51, 2550–2562; Angew. Chem. 2012, 124, 2600–2612.