Enantioselective Reductive Cyanation and Phosphonylation of Secondary Amides by Iridium and Chiral Thiourea Sequential Catalysis
Dong-Huang Chen
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorWei-Ting Sun
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorCheng-Jie Zhu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorGuang-Sheng Lu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
Search for more papers by this authorDong-Ping Wu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
Search for more papers by this authorCorresponding Author
Dr. Ai-E Wang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, Gansu China
Search for more papers by this authorCorresponding Author
Prof. Dr. Pei-Qiang Huang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, Gansu China
Search for more papers by this authorDong-Huang Chen
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorWei-Ting Sun
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorCheng-Jie Zhu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
These authors contributed equally to this work.
Search for more papers by this authorGuang-Sheng Lu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
Search for more papers by this authorDong-Ping Wu
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
Search for more papers by this authorCorresponding Author
Dr. Ai-E Wang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, Gansu China
Search for more papers by this authorCorresponding Author
Prof. Dr. Pei-Qiang Huang
Department of Chemistry and Fujian Provincial Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005 Fujian, China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, 730000, Gansu China
Search for more papers by this authorGraphical Abstract
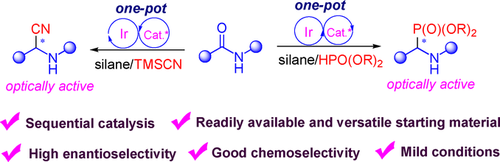
The first enantioselective reductive cyanation and phosphonylation of secondary amides have been achieved by the combination of iridium with chiral thiourea catalysis. The protocol is highly efficient and enantioselective, providing a novel route for the synthesis of optically active α-aminonitriles and α-aminophosphonates from bench-stable feedstocks.
Abstract
The combination of transition-metal catalysis and organocatalysis increasingly offers chemists opportunities to realize diverse unprecedented chemical transformations. By combining iridium with chiral thiourea catalysis, direct enantioselective reductive cyanation and phosphonylation of secondary amides have been accomplished for the first time for the synthesis of enantioenriched chiral α-aminonitriles and α-aminophosphonates. The protocol is highly efficient and enantioselective, providing a novel route to the synthesis of optically active α-functionalized amines from the simple, readily available feedstocks. In addition, the reactions are scalable and the thiourea catalyst can be recycled and reused.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202015898-sup-0001-misc_information.pdf7.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aF. F. Fleming, Nat. Prod. Rep. 1999, 16, 597;
- 1bF. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk, B. C. Shook, J. Med. Chem. 2010, 53, 7902;
- 1cV. V. Kouznetsov, C. E. Puerto Galvis, Tetrahedron 2018, 74, 773.
- 2
- 2aP. Kafarski, B. Lejczak, Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 193;
- 2b Aminophosphonic and aminophosphinic acids: chemistry and biological activity (Eds.: V. P. Kukhar, H. R. Hudson), Wiley, Chichester, 2000;
- 2cM. Sieńczyk, J. Oleksyszyn, Curr. Med. Chem. 2009, 16, 1673;
- 2dF. Orsini, G. Sello, M. Sisti, Curr. Med. Chem. 2010, 17, 264;
- 2eA. Mucha, P. Kafarski, Ł. Berlicki, J. Med. Chem. 2011, 54, 5955.
- 3For reviews, see:
- 3aJ. Wang, X. Liu, X. Feng, Chem. Rev. 2011, 111, 6947;
- 3bX.-H. Cai, B. Xie, ARKIVOC 2014, 205;
- 3cN. Kurono, T. Ohkuma, ACS Catal. 2016, 6, 989; for selected examples, see:
- 3dM. S. Sigman, E. N. Jacobsen, J. Am. Chem. Soc. 1998, 120, 4901;
- 3eP. Vachal, E. N. Jacobsen, J. Am. Chem. Soc. 2002, 124, 10012;
- 3fM. Rueping, E. Sugiono, C. Azap, Angew. Chem. Int. Ed. 2006, 45, 2617; Angew. Chem. 2006, 118, 2679;
- 3gS. J. Zuend, M. P. Coughlin, M. P. Lalonde, E. N. Jacobsen, Nature 2009, 461, 968;
- 3hS. J. Zuend, E. N. Jacobsen, J. Am. Chem. Soc. 2009, 131, 15358.
- 4For a review, see:
- 4aP. Merino, E. Marqués-Lépez, R. P. Herrera, Adv. Synth. Catal. 2008, 350, 1195; for selected examples, see:
- 4bG. D. Joly, E. N. Jacobsen, J. Am. Chem. Soc. 2004, 126, 4102;
- 4cT. Akiyama, H. Morita, J. Itoh, K. Fuchibe, Org. Lett. 2005, 7, 2583;
- 4dY. Zhao, X. Li, F. Mo, L. Li, X. Lin, RSC Adv. 2013, 3, 11895.
- 5
- 5aS. C. Pan, B. List, Org. Lett. 2007, 9, 1149;
- 5bY. H. Wen, Y. Xiong, L. Chang, J. L. Huang, X. H. Liu, X. M. Feng, J. Org. Chem. 2007, 72, 7715;
- 5cY. H. Wen, B. Gao, Y. Z. Fu, S. X. Dong, X. H. Liu, X. M. Feng, Chem. Eur. J. 2008, 14, 6789.
- 6
- 6aX. Cheng, R. Goddard, G. Buth, B. List, Angew. Chem. Int. Ed. 2008, 47, 5079; Angew. Chem. 2008, 120, 5157;
- 6bX. Zhou, D. J. Shang, Q. Zhang, L. L. Lin, X. H. Liu, X. M. Feng, Org. Lett. 2009, 11, 1401;
- 6cM. Ohara, S. Nakamura, N. Shibata, Adv. Synth. Catal. 2011, 353, 3285;
- 6dL. Zou, J. Huang, N. Liao, Y. Liu, Q. Guo, Y. Peng, Org. Lett. 2020, 22, 6932.
- 7For a review see: M. Ordóñez, J. Viveros-Ceballos, C. Cativiela, F. J. Sayago, Tetrahedron 2015, 71, 1745.
- 8 The Amide Linkage: Structural Significance in Chemistry, Biochemistry and Materials Science (Eds.: A. Greenberg, C. M. Breneman, J. F. Liebman), Wiley, New York, 2000.
- 9D. Eisenberg, Proc. Natl. Acad. Sci. USA 2003, 100, 11207.
- 10K. Marchildon, Macromol. React. Eng. 2011, 5, 22.
- 11
- 11aR.-Y. Zhu, M. E. Farmer, Y.-Q. Chen, J.-Q. Yu, Angew. Chem. Int. Ed. 2016, 55, 10578; Angew. Chem. 2016, 128, 10734;
- 11bC. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes, M. Schnürch, Chem. Soc. Rev. 2018, 47, 6603;
- 11cT. G. Saint-Denis, R.-Y. Zhu, G. Chen, Q.-F. Wu, J.-Q. Yu, Science 2018, 359, eaao4798.
- 12For selected examples, see:
- 12aG. Pelletier, W. S. Bechara, A. B. Charette, J. Am. Chem. Soc. 2010, 132, 12817;
- 12bW. S. Bechara, G. Pelletier, A. B. Charette, Nat. Chem. 2012, 4, 228;
- 12cG. Pelletier, A. B. Charette, Org. Lett. 2013, 15, 2290;
- 12dW. S. Bechara, I. S. Khazhieva, E. Rodriguez, A. B. Charette, Org. Lett. 2015, 17, 1184.
- 13For selected examples, see:
- 13aM. Movassaghi, M. D. Hill, O. K. Ahmad, J. Am. Chem. Soc. 2007, 129, 10096;
- 13bM. Movassaghi, M. D. Hill, Org. Lett. 2008, 10, 3485;
- 13cO. K. Ahmad, M. D. Hill, M. Movassaghi, J. Org. Chem. 2009, 74, 8460.
- 14For selected examples, see:
- 14aY. Oda, T. Sato, N. Chida, Org. Lett. 2012, 14, 950;
- 14bM. Nakajima, Y. Oda, T. Wada, R. Minamikawa, K. Shirokane, T. Sato, N. Chida, Chem. Eur. J. 2014, 20, 17565.
- 15
- 15aP.-Q. Huang, Y.-H. Huang, H. Geng, J.-L. Ye, Sci. Rep. 2016, 6, 28801;
- 15bT. Fan, A. Wang, J.-Q. Li, J.-L. Ye, X. Zheng, P.-Q. Huang, Angew. Chem. Int. Ed. 2018, 57, 10352; Angew. Chem. 2018, 130, 10509.
- 16For selected recent contributions from other groups, see:
- 16aV. Tona, B. Maryasin, A. de la Torre, J. Sprachmann, L. González, N. Maulide, Org. Lett. 2017, 19, 2662;
- 16bJ. Li, R. Oost, B. Maryasin, L. González, N. Maulide, Nat. Commun. 2019, 10, 2327;
- 16cW. Sun, L. Wang, Y. Hu, X. Wu, C. Xia, C. Liu, Nat. Commun. 2020, 11, 3113.
- 17For reviews, see:
- 17aD. Seebach, Angew. Chem. Int. Ed. 2011, 50, 96; Angew. Chem. 2011, 123, 99;
- 17bV. Pace, W. Holzer, Aust. J. Chem. 2013, 66, 507;
- 17cV. Pace, W. Holzer, B. Olofsson, Adv. Synth. Catal. 2014, 356, 3697;
- 17dT. Sato, N. Chida, Org. Biomol. Chem. 2014, 12, 3147;
- 17eD. Kaiser, N. Maulide, J. Org. Chem. 2016, 81, 4421;
- 17fT. Sato, M. Yoritate, H. Tajima, N. Chida, Org. Biomol. Chem. 2018, 16, 3864;
- 17gD. Kaiser, A. Bauer, M. Lemmerera, N. Maulide, Chem. Soc. Rev. 2018, 47, 7899;
- 17hP.-Q. Huang, Acta Chim. Sinica 2018, 76, 357.
- 18
- 18aK.-J. Xiao, A.-E Wang, P.-Q. Huang, Angew. Chem. Int. Ed. 2012, 51, 8314; Angew. Chem. 2012, 124, 8439;
- 18bP.-Q. Huang, Y.-H. Huang, K.-J. Xiao, Y. Wang, X.-E. Xia, J. Org. Chem. 2015, 80, 2861;
- 18cA.-E Wang, C.-C. Yu, Y.-P. Liu, P.-Q. Huang, Org. Lett. 2018, 20, 999;
- 18dZ. Xu, X.-G. Wang, Y.-H. Wei, K.-L. Ji, J.-F. Zheng, J.-L. Ye, P.-Q. Huang, Org. Lett. 2019, 21, 7587.
- 19
- 19aQ. Xia, B. Ganem, Org. Lett. 2001, 3, 485;
- 19bY. Z. Gao, Z. B. Huang, R. Q. Zhuang, J. Xu, P. B. Zhang, G. Tang, Y. F. Zhao, Org. Lett. 2013, 15, 4214;
- 19cJ.-F. Zheng, X.-N. Hu, Z. Xu, D.-C. Cai, T.-L. Shen, P.-Q. Huang, J. Org. Chem. 2017, 82, 9693.
- 20C. Cheng, M. Brookhart, J. Am. Chem. Soc. 2012, 134, 11304.
- 21
- 21aW. Ou, F. Han, X.-N. Hu, H. Chen, P.-Q. Huang, Angew. Chem. Int. Ed. 2018, 57, 11354; Angew. Chem. 2018, 130, 11524;
- 21bY. Takahashi, R. Yoshii, T. Sato, N. Chida, Org. Lett. 2018, 20, 5705.
- 22For Ir-catalyzed racemic reductive functionalization of tertiary amides, see:
- 22aA. W. Gregory, A. Chambers, A. Hawkins, P. Jakubec, D. J. Dixon, Chem. Eur. J. 2015, 21, 111;
- 22bM. Nakajima, T. Sato, N. Chida, Org. Lett. 2015, 17, 1696;
- 22cA. L. Fuentes de Arriba, E. Lenci, M. Sonawane, O. Formery, D. J. Dixon, Angew. Chem. Int. Ed. 2017, 56, 3655; Angew. Chem. 2017, 129, 3709;
- 22dY. Takahashi, T. Sato, N. Chida, Chem. Lett. 2019, 48, 1138; For a revew, see:
- 22eD. Matheau-Raven, P. Gabriel, J. A. Leitch, Y. A. Almehmadi, K. Yamazaki, D. J. Dixon, ACS Catal. 2020, 10, 8880.
- 23For selected reviews, see:
- 23aZ. Du, Z. Shao, Chem. Soc. Rev. 2013, 42, 1337;
- 23bD.-F. Chen, Z.-Y. Han, X.-L. Zhou, L.-Z. Gong, Acc. Chem. Res. 2014, 47, 2365;
- 23cS. Afewerki, A. Clrdova, Chem. Rev. 2016, 116, 13512; For selected examples, see:
- 23dK. Namitharan, T. Zhu, J. Cheng, P. Zheng, X. Li, S. Yang, B. Song, Y. R. Chi, Nat. Commun. 2014, 5, 3982;
- 23eL. Zhou, X. Wu, X. Yang, C. Mou, R. Song, S. Yu, H. Chai, L. Pan, Z. Jin, Y. R. Chi, Angew. Chem. Int. Ed. 2020, 59, 1557; Angew. Chem. 2020, 132, 1573;
- 23fY. Kuang, K. Wang, X. Shi, X. Huang, E. Meggers, J. Wu, Angew. Chem. Int. Ed. 2019, 58, 16859; Angew. Chem. 2019, 131, 17015.
- 24
- 24aM. Terada, Chem. Commun. 2008, 4097;
- 24bM. Terada, Synthesis 2010, 1929;
- 24cD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 24dT. Akiyama, K. Mori, Chem. Rev. 2015, 115, 9277.
- 25
- 25aY. Takemoto, Org. Biomol. Chem. 2005, 3, 4299;
- 25bZ. Zhang, P. R. Schreiner, Chem. Soc. Rev. 2009, 38, 1187;
- 25cO. V. Serdyuk, C. M. Heckel, S. B. Tsogoeva, Org. Biomol. Chem. 2013, 11, 7051;
- 25dX. Fang, C.-J. Wang, Chem. Commun. 2015, 51, 1185.
- 26C. C. Beard, M. B. Wallach, K. Weinhardt (Syntex Inc.), US 4110463, 1978.
- 27B. Badet, C. Walsh, Biochemistry 1985, 24, 1333.
- 28
- 28aJ. G. Allen, F. R. Atherton, M. J. Hall, C. H. Hassall, S. W. Holmes, R. W. Lambert, L. J. Nisbet, P. S. Ringrose, Nature 1978, 272, 56;
- 28bF. R. Atherton, C. H. Hassall, R. W. Lambert, J. Med. Chem. 1986, 29, 29.