Macrocyclic Arenes-Based Conjugated Macrocycle Polymers for Highly Selective CO2 Capture and Iodine Adsorption
Dihua Dai
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorJie Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Yong-Cun Zou
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorJia-Rui Wu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Li-Li Tan
State Key Laboratory of Solidification Processing, Center for Nano Energy Materials, School of Materials Science and Engineering, Northwestern Polytechnical University and Shaanxi Joint Laboratory of Graphene (NPU), 127 Youyi West Road, Xi'an, 710072 P. R. China
Search for more papers by this authorProf. Yan Wang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bao Li
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorDr. Tong Lu
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bo Wang
Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Key Laboratory of Cluster Science, Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ying-Wei Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorDihua Dai
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorJie Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Yong-Cun Zou
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorJia-Rui Wu
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Li-Li Tan
State Key Laboratory of Solidification Processing, Center for Nano Energy Materials, School of Materials Science and Engineering, Northwestern Polytechnical University and Shaanxi Joint Laboratory of Graphene (NPU), 127 Youyi West Road, Xi'an, 710072 P. R. China
Search for more papers by this authorProf. Yan Wang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bao Li
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorDr. Tong Lu
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorProf. Bo Wang
Beijing Key Laboratory of Photoelectronic/Electrophotonic Conversion Materials, Key Laboratory of Cluster Science, Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081 P. R. China
Search for more papers by this authorCorresponding Author
Prof. Ying-Wei Yang
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry, College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012 P. R. China
Search for more papers by this authorGraphical Abstract
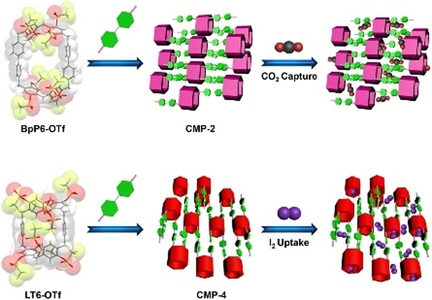
Four catcher-type conjugated macrocycle polymers (CMP-n, n=1–4) have been designed and synthesized successfully, exhibiting interesting application in CO2 and I2 uptake. The CMP-2 is able to capture CO2 with excellent selectivity and the CMP-4 is capable of adsorbing iodine with outstanding capacity.
Abstract
Incorporating synthetic macrocycles with unique structures and distinct conformations into conjugated macrocycle polymers (CMPs) can endow the resulting materials with great potentials in gas uptake and pollutant adsorption. Here, four CMPs (CMP-n, n=1–4) capable of reversibly capturing iodine and efficiently separating carbon dioxide are constructed from per-triflate functionalized leaning tower[6]arene (LT6-OTf) and [2]biphenyl-extended pillar[6]arene (BpP6-OTf) via Pd-catalyzed Sonogashira–Hagihara cross-coupling reaction. Intriguingly, owing to the appropriate cavity size of LT6-OTf and the numerous aromatic rings in the framework, the newly designed CMP-4 possesses an outstanding I2 affinity with a large uptake capacity of 208 wt % in vapor and a great removal efficiency of 94 % in aqueous solutions. To our surprise, with no capacity to accommodate nitrogen, CMP-2 constructed from BpP6-OTf is able to specifically capture carbon dioxide at ambient conditions.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202015162-sup-0001-cif.zip1.3 MB | Supplementary |
| anie202015162-sup-0001-misc_information.pdf9.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger, O. M. Yaghi, Science 2005, 310, 1166–1170;
- 1bC. Qian, Q. Y. Qi, G. F. Jiang, F. Z. Cui, Y. Tian, X. Zhao, J. Am. Chem. Soc. 2017, 139, 6736–6743;
- 1cP. Wang, Q. Xu, Z. Li, W. Jiang, Q. Jiang, D. Jiang, Adv. Mater. 2018, 30, 1801991.
- 2
- 2aH.-L. Li, M. Eddaoudi, M. O'Keeffe, O. M. Yaghi, Nature 1999, 402, 276–279;
- 2bJ. A. Mason, J. Oktawiec, M. K. Taylor, M. R. Hudson, J. Rodriguez, J. E. Bachman, M. I. Gonzalez, A. Cervellino, A. Guagliardi, C. M. Brown, P. L. Llewellyn, N. Masciocchi, J. R. Long, Nature 2015, 527, 357–361;
- 2cQ. Wang, X. Feng, S. Wang, N. Song, Y. Chen, W. Tong, Y. Han, L. Yang, B. Wang, Adv. Mater. 2016, 28, 5837–5843;
- 2dA. Kirchon, L. Feng, H. F. Drake, E. A. Joseph, H.-C. Zhou, Chem. Soc. Rev. 2018, 47, 8611–8638.
- 3
- 3aJ. X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak, A. I. Cooper, Angew. Chem. Int. Ed. 2007, 46, 8574–8578; Angew. Chem. 2007, 119, 8728–8732;
- 3bY. Xu, S. Jin, H. Xu, A. Nagai, D. Jiang, Chem. Soc. Rev. 2013, 42, 8012–8031.
- 4
- 4aB. Aguila, Q. Sun, J. A. Perman, L. D. Earl, C. W. Abney, R. Elzein, R. Schlaf, S. Ma, Adv. Mater. 2017, 29, 1700665;
- 4bL. Zou, Y. Sun, S. Che, X. Yang, X. Wang, M. Bosch, Q. Wang, H. Li, M. Smith, S. Yuan, Z. Perry, H. C. Zhou, Adv. Mater. 2017, 29, 1700229.
- 5S. N. Talapaneni, D. Kim, G. Barin, O. Buyukcakir, S. H. Je, A. Coskun, Chem. Mater. 2016, 28, 4460–4466.
- 6Q. Sun, B. Aguila, J. Perman, L. D. Earl, C. W. Abney, Y. Cheng, H. Wei, N. Nguyen, L. Wojtas, S. Ma, J. Am. Chem. Soc. 2017, 139, 2786–2793.
- 7J. Lee, O. Buyukcakir, T. W. Kwon, A. Coskun, J. Am. Chem. Soc. 2018, 140, 10937–10940.
- 8H. Ma, B. Liu, B. Li, L. Zhang, Y. G. Li, H. Q. Tan, H. Y. Zang, G. Zhu, J. Am. Chem. Soc. 2016, 138, 5897–5903.
- 9Z. Li, Y.-W. Yang, J. Mater. Chem. B 2017, 5, 9278–9290.
- 10S. Haldar, D. Chakraborty, B. Roy, G. Banappanavar, K. Rinku, D. Mullangi, P. Hazra, D. Kabra, R. Vaidhyanathan, J. Am. Chem. Soc. 2018, 140, 13367–13374.
- 11S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Su, W. Wang, J. Am. Chem. Soc. 2011, 133, 19816–19822.
- 12
- 12aY. Lin, X. Jiang, S. T. Kim, S. B. Alahakoon, X. Hou, Z. Zhang, C. M. Thompson, R. A. Smaldone, C. Ke, J. Am. Chem. Soc. 2017, 139, 7172–7175;
- 12bD. Shetty, J. Raya, D. S. Han, Z. Asfari, J.-C. Olsen, A. Trabolsi, Chem. Mater. 2017, 29, 8968–8972;
- 12cM. Janeta, W. Bury, S. Szafert, ACS Appl. Mater. Interfaces 2018, 10, 19964–19973;
- 12dB. Li, B. Wang, X. Huang, L. Dai, L. Cui, J. Li, X. Jia, C. Li, Angew. Chem. Int. Ed. 2019, 58, 3885–3889; Angew. Chem. 2019, 131, 3925–3929.
- 13X. Li, Z. Li, Y.-W. Yang, Adv. Mater. 2018, 30, 1800177.
- 14Z. Li, X. Li, Y.-W. Yang, Small 2019, 15, 1805509.
- 15
- 15aT. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 2008, 130, 5022–5023;
- 15bD. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang, H. Meier, Angew. Chem. Int. Ed. 2009, 48, 9721–9723; Angew. Chem. 2009, 121, 9901–9903;
- 15cX. B. Hu, Z. Chen, L. Chen, L. Zhang, J. L. Hou, Z. T. Li, Chem. Commun. 2012, 48, 10999–11001;
- 15dK. Jie, Y. Zhou, E. Li, Z. Li, R. Zhao, F. Huang, J. Am. Chem. Soc. 2017, 139, 15320–15323;
- 15eS. Wang, Z. Xu, T. Wang, T. Xiao, X. Y. Hu, Y. Z. Shen, L. Wang, Nat. Commun. 2018, 9, 1737;
- 15fJ. Chen, H. Ni, Z. Meng, J. Wang, X. Huang, Y. Dong, C. Sun, Y. Zhang, L. Cui, J. Li, X. Jia, Q. Meng, C. Li, Nat. Commun. 2019, 10, 3546;
- 15gX.-H. Wang, N. Song, W. Hou, C.-Y. Wang, Y. Wang, J. Tang, Y.-W. Yang, Adv. Mater. 2019, 31, 1903962.
- 16B. Gao, L.-L. Tan, N. Song, K. Li, Y.-W. Yang, Chem. Commun. 2016, 52, 5804–5807.
- 17
- 17aJ.-R. Wu, C.-Y. Wang, Y.-C. Tao, Y. Wang, C. Li, Y.-W. Yang, Eur. J. Org. Chem. 2018, 1321–1325;
- 17bD. Dai, Z. Li, J. Yang, C. Wang, J. R. Wu, Y. Wang, D. Zhang, Y.-W. Yang, J. Am. Chem. Soc. 2019, 141, 4756–4763.
- 18
- 18aD. Shetty, A. Trabolsi, Sci. China Chem. 2019, 62, 289–290;
- 18bT. M. Swager, Y. Kim, Synfacts 2018, 14, 0815;
10.1055/s-0037-1610512 Google Scholar
- 18cJ.-R. Wu, A. U. Mu, B. Li, C. Y. Wang, L. Fang, Y.-W. Yang, Angew. Chem. Int. Ed. 2018, 57, 9853–9858; Angew. Chem. 2018, 130, 10001–10006.
- 19
- 19aZ.-J. Liu, J.-R. Wu, C.-Y. Wang, J. Yang, Y. Wang, Y.-W. Yang, Chin. Chem. Lett. 2019, 30, 2299–2303;
- 19bJ.-R. Wu, Y.-W. Yang, Chem. Commun. 2019, 55, 1533–1543.
- 20
- 20aJ. T. Litynski, S. M. Klara, H. G. McIlvried, R. D. Srivastava, Environ. Int. 2006, 32, 128–144;
- 20bS. Fuss, J. G. Canadell, G. P. Peters, M. Tavoni, R. M. Andrew, P. Ciais, R. B. Jackson, C. D. Jones, F. Kraxner, N. Nakicenovic, C. Le Quéré, M. R. Raupach, A. Sharifi, P. Smith, Y. Yamagata, Nat. Clim. Change 2014, 4, 850–853;
- 20cY. Yang, F. Jiang, L. Chen, J. Pang, M. Wu, X. Wan, J. Pan, J. Qian, M. Hong, J. Mater. Chem. A 2015, 3, 13526–13532.
- 21
- 21aA. Aguilar-Granda, S. Perez-Estrada, E. Sanchez-Gonzalez, J. R. Alvarez, J. Rodriguez-Hernandez, M. Rodriguez, A. E. Roa, S. Hernandez-Ortega, I. A. Ibarra, B. Rodriguez-Molina, J. Am. Chem. Soc. 2017, 139, 7549–7557;
- 21bM. Arici, O. Z. Yeşilel, M. Taş, H. Demiral, Cryst. Growth Des. 2017, 17, 2654–2659;
- 21cA. Chaix, G. Mouchaham, A. Shkurenko, P. Hoang, B. Moosa, P. M. Bhatt, K. Adil, K. N. Salama, M. Eddaoudi, N. M. Khashab, J. Am. Chem. Soc. 2018, 140, 14571–14575;
- 21dD. Chen, Y. Fu, W. Yu, G. Yu, C. Pan, Chem. Eng. J. 2018, 334, 900–906;
- 21eS. T. Wang, J. L. Hong, ACS Omega 2019, 4, 12018–12027.
- 22
- 22aW. Lee, M. Ojovan, M. Stennett, N. Hyatt, Adv. Appl. Ceram. 2006, 105, 3–12;
- 22bF. C. Küpper, M. C. Feiters, B. Olofsson, T. Kaiho, S. Yanagida, M. B. Zimmermann, L. J. Carpenter, G. W. Luther, Z. Lu, M. Jonsson, L. Kloo, Angew. Chem. Int. Ed. 2011, 50, 11598–11620; Angew. Chem. 2011, 123, 11802–11825;
- 22cA. Saiz-Lopez, J. M. C. Plane, A. R. Baker, L. J. Carpenter, R. von Glasow, J. C. G. Martin, G. McFiggans, R. W. Saunders, Chem. Rev. 2012, 112, 1773–1804.
- 23
- 23aS. A, Y. Zhang, Z. Li, H. Xia, M. Xue, X. Liu, Y. Mu, Chem. Commun. 2014, 50, 8495–8498;
- 23bS. Ma, S. M. Islam, Y. Shim, Q. Gu, P. Wang, H. Li, G. Sun, X. Yang, M. G. Kanatzidis, Chem. Mater. 2014, 26, 7114–7123;
- 23cX. Jiang, X. Cui, A. J. E. Duncan, L. Li, R. P. Hughes, R. J. Staples, E. V. Alexandrov, D. M. Proserpio, Y. Wu, C. Ke, J. Am. Chem. Soc. 2019, 141, 10915–10923;
- 23dJ.-R. Wu, B. Li, J.-W. Zhang, Y.-W. Yang, ACS Appl. Mater. Interfaces 2019, 11, 998–1003.
- 24
- 24aL.-L. Tan, H. Li, Y. Tao, S. X. Zhang, B. Wang, Y.-W. Yang, Adv. Mater. 2014, 26, 7027–7031;
- 24bL.-L. Tan, Y. Zhou, Y. Jin, W. Zhang, Y.-W. Yang, Supramol. Chem. 2018, 30, 648–654;
- 24cZ. V. Singh, L.-L. Tan, M. G. Cowan, Y.-W. Yang, W. Zhang, D. L. Gin, R. D. Noble, J. Membr. Sci. 2017, 539, 224–228;
- 24dL.-L. Tan, Y. Zhou, H. Long, Y. Jin, W. Zhang, Y.-W. Yang, Chem. Commun. 2017, 53, 6409–6412.
- 25Y. Li, Y. Segawa, A. Yagi, K. Itami, J. Am. Chem. Soc. 2020, 142, 12850–12856.
- 26
- 26aK. Wang, C.-Y. Wang, Y. Wang, H. Li, C.-Y. Bao, J.-Y. Liu, S. X.-A. Zhang, Y.-W. Yang, Chem. Commun. 2013, 49, 10528–10530;
- 26bK. Wang, C.-Y. Wang, Y. Zhang, S. X.-A. Zhang, B. Yang, Y.-W. Yang, Chem. Commun. 2014, 50, 9458–9461.
- 27D. Shetty, T. Skorjanc, J. Raya, S. K. Sharma, I. Jahovic, K. Polychronopoulou, Z. Asfari, D. S. Han, S. Dewage, J. C. Olsen, R. Jagannathan, S. Kirmizialtin, A. Trabolsi, ACS Appl. Mater. Interfaces 2018, 10, 17359–17365.
- 28
- 28aZ. Wang, Y. Huang, J. Yang, Y. Li, Q. Zhuang, J. Gu, Dalton Trans. 2017, 46, 7412–7420;
- 28bC. Pei, T. Ben, S. Xu, S. Qiu, J. Mater. Chem. A 2014, 2, 7179–7187;
- 28cH. Ma, J.-J. Chen, L. Tan, J.-H. Bu, Y. Zhu, B. Tan, C. Zhang, ACS Macro Lett. 2016, 5, 1039–1043;
- 28dT. Hasell, M. Schmidtmann, A. I. Cooper, J. Am. Chem. Soc. 2011, 133, 14920–14923.
- 29H. A. Benesi, J. H. Hildebrand, J. Am. Chem. Soc. 1949, 71, 2703–2707.
- 30
- 30aJ. Tian, P. K. Thallapally, S. J. Dalgarno, J. L. Atwood, J. Am. Chem. Soc. 2009, 131, 13216–13217;
- 30bR. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe, O. M. Yaghi, Science 2008, 319, 939–943;
- 30cD. Liu, J. Gu, Q. Liu, Y. Tan, Z. Li, W. Zhang, Y. Su, W. Li, A. Cui, C. Gu, D. Zhang, Adv. Mater. 2014, 26, 1229–1234;
- 30dB. Wang, A. P. Cote, H. Furukawa, M. O'Keeffe, O. M. Yaghi, Nature 2008, 453, 207–211.
- 31
- 31aW.-G. Cui, T.-L. Hu, X.-H. Bu, Adv. Mater. 2020, 32, 1806445;
- 31bD. M. D'Alessandro, B. Smit, R. L. Jeffrey, Angew. Chem. Int. Ed. 2010, 49, 6058–6082; Angew. Chem. 2010, 122, 6194–6219.
- 32
- 32aY. Jiang, P. Tan, S.-C. Qi, X.-Q. Liu, J.-H. Yan, F. Fan, L.-B. Sun, Angew. Chem. Int. Ed. 2019, 58, 6600–6604; Angew. Chem. 2019, 131, 6672–6676;
- 32bN. C. Burtch, H. Jasuja, K. S. Walton, Chem. Rev. 2014, 114, 10575–10612.
- 33Deposition Numbers 1965412 and 1965414 (for LT6-OTf and BpP6-OTf) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.