Iron-Catalyzed Radical Activation Mechanism for Denitrogenative Rearrangement Over C(sp3)–H Amination
Satyajit Roy
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005 India
These authors contributed equally to this work.
Search for more papers by this authorSandip Kumar Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorHillol Khatua
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorSubrata Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorProf. Dr. Krishna Nand Singh
Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorSatyajit Roy
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005 India
These authors contributed equally to this work.
Search for more papers by this authorSandip Kumar Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
These authors contributed equally to this work.
Search for more papers by this authorHillol Khatua
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorSubrata Das
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorProf. Dr. Krishna Nand Singh
Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, 221005 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Buddhadeb Chattopadhyay
Division of Molecular Synthesis & Drug Discovery, Centre of Bio-Medical Research (CBMR), SGPGIMS Campus, Raebareli Road, Lucknow, 226014 U.P., India
Search for more papers by this authorGraphical Abstract
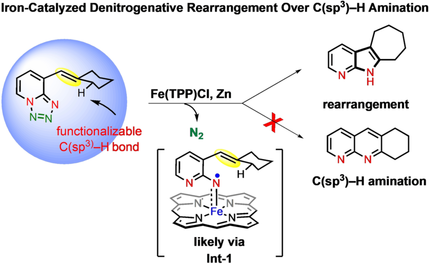
An iron-catalyzed rearrangement of 1,2,3,4-tetrazole is developed over the competitive C(sp3)–H amination. This catalytic rearrangement follows an unprecedented metalloradical activation mechanism. Employing the developed method, a wide number of complex-N-heterocyclic product classes have been accessed.
Abstract
An iron-catalyzed denitrogenative rearrangement of 1,2,3,4-tetrazole is developed over the competitive C(sp3)–H amination. This catalytic rearrangement reaction follows an unprecedented metalloradical activation mechanism. Employing the developed method, a wide number of complex-N-heterocyclic product classes have been accessed. The synthetic utility of this radical activation method is showcased with the short synthesis of a bioactive molecule. Collectively, this discovery underlines the progress of radical activation strategy that should find wide application in the perspective of medicinal chemistry, drug discovery and natural product synthesis research.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202014950-sup-0001-misc_information.pdf11.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. V. Smirnov, D. S. English, R. L. Rich, J. Lane, L. Teyton, A. W. Schwabacher, S. Luo, R. W. Thornburg, J. W. Petrich, J. Phys. Chem. B 1997, 101, 2758–2769;
- 1bS. B. Zhao, S. Wang, Chem. Soc. Rev. 2010, 39, 3142–3156.
- 2M. F. Richter, B. S. Drown, A. P. Riley, A. Garcia, T. Shirai, R. L. Svec, P. J. Hergenrother, Nature 2017, 545, 299–304.
- 3P. Dowd, W. Zhang, Chem. Rev. 1993, 93, 2091–2115.
- 4A. L. J. Beckwith, D. M. O'Shea, S. W. Westwood, J. Am. Chem. Soc. 1988, 110, 2565–2575.
- 5T. Miura, Y. Funakoshi, M. Morimoto, T. Biyajima, M. Murakami, J. Am. Chem. Soc. 2012, 134, 17440–17443.
- 6N. Selander, B. T. Worrell, V. V. Fokin, Angew. Chem. Int. Ed. 2012, 51, 13054–13057; Angew. Chem. 2012, 124, 13231–13234.
- 7K. Sun, S. Liu, P. M. Bec, T. G. Driver, Angew. Chem. Int. Ed. 2011, 50, 1702–1706; Angew. Chem. 2011, 123, 1740–1744.
- 8
- 8aK. Chen, Z.-Z. Zhu, Y.-S. Zhang, X.-Y. Tang, M. Shi, Angew. Chem. Int. Ed. 2014, 53, 6645–6649; Angew. Chem. 2014, 126, 6763–6767;
- 8bK. Chen, Z.-Z. Zhu, J.-X. Liu, X.-Y. Tang, Y. Wei, M. Shi, Chem. Commun. 2016, 52, 350–353.
- 9B. Chattopadhyay, C. I. Rivera Vera, S. Chuprakov, V. Gevorgyan, Org. Lett. 2010, 12, 2166–2169.
- 10
- 10aH. Lu, H. Jiang, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2010, 49, 10192–10196; Angew. Chem. 2010, 122, 10390–10394;
- 10bY. Liu, J. Wei, C.-M. Che, Chem. Commun. 2010, 46, 6926–6928;
- 10cS. M. Paradine, M. C. White, J. Am. Chem. Soc. 2012, 134, 2036–2039;
- 10dE. T. Hennessy, T. A. Betley, Science 2013, 340, 591–595;
- 10eY. Liu, X. Guan, E. L.-M. Wong, P. Liu, J.-S. Huang, C.-M. Che, J. Am. Chem. Soc. 2013, 135, 7194–7204;
- 10fH. Lu, C. Li, H. Jiang, C. L. Lizardi, X. P. Zhang, Angew. Chem. Int. Ed. 2014, 53, 7028–7032; Angew. Chem. 2014, 126, 7148–7152;
- 10gS. M. Paradine, J. R. Griffin, J. Zhao, A. L. Petronico, S. M. Miller, M. C. White, Nat. Chem. 2015, 7, 987–994;
- 10hH. Lu, K. Lang, H. Jiang, L. Wojtas, X. P. Zhang, Chem. Sci. 2016, 7, 6934–6939;
- 10iI. T. Alt, B. Plietker, Angew. Chem. Int. Ed. 2016, 55, 1519–1522; Angew. Chem. 2016, 128, 1542–1545;
- 10jB. Bagh, D. L. J. Broere, V. Sinha, P. F. Kuijpers, N. P. van Leest, B. de Bruin, S. Demeshko, M. A. Siegler, J. I. van der Vlugt, J. Am. Chem. Soc. 2017, 139, 5117–5124;
- 10kI. T. Alt, C. Guttroff, B. Plietker, Angew. Chem. Int. Ed. 2017, 56, 10582–10586; Angew. Chem. 2017, 129, 10718–10722;
- 10lC. Li, K. Lang, H. Lu, Y. Hu, X. Cui, L. Wojtas, X. P. Zhang, Angew. Chem. Int. Ed. 2018, 57, 16837–16841; Angew. Chem. 2018, 130, 17079–17083;
- 10mK.-P. Shing, Y. Liu, B. Cao, X.-Y. Chang, T. You, C.-M. Che, Angew. Chem. Int. Ed. 2018, 57, 11947–11951; Angew. Chem. 2018, 130, 12123–12127;
- 10nB. Plietker, A. Röske, Catal. Sci. Technol. 2019, 9, 4188–4197;
- 10oS. Liang, X. Zhao, T. Yang, W. Yu, Org. Lett. 2020, 22, 1961–1965;
- 10pM. Zhou, M. Lankelma, J. I. van der Vlugt, B. de Bruin, Angew. Chem. Int. Ed. 2020, 59, 2–9; Angew. Chem. 2020, 132, 11073–11079.
- 11H. M. L. Davies, J. R. Manning, Nature 2008, 451, 417–424.
- 12
- 12aS.-M. Au, J.-S. Huang, W.-Y. Yu, W.-H. Fung, C.-M. Che, J. Am. Chem. Soc. 1999, 121, 9120–9132;
- 12bB. J. Stokes, H. Dong, B. E. Leslie, A. L. Pumphrey, T. G. Driver, J. Am. Chem. Soc. 2007, 129, 7500–7501;
- 12cC. Liang, F. Collet, F. Robert-Peillard, P. Muller, R. H. Dodd, P. Dauban, J. Am. Chem. Soc. 2008, 130, 343–350;
- 12dM. Shen, B. E. Leslie, T. G. Driver, Angew. Chem. Int. Ed. 2008, 47, 5056–5059; Angew. Chem. 2008, 120, 5134–5137;
- 12eD. N. Zalatan, J. Du Bois, Top. Curr. Chem. 2010, 292, 347–378;
- 12fT. G. Driver, Org. Biomol. Chem. 2010, 8, 3831–3846;
- 12gB. J. Stokes, S. Liu, T. G. Driver, J. Am. Chem. Soc. 2011, 133, 4702–4705;
- 12hM. E. Harvey, D. G. Musaev, J. Du Bois, J. Am. Chem. Soc. 2011, 133, 17207–17216;
- 12iA. L. Pumphrey, H. Dong, T. G. Driver, Angew. Chem. Int. Ed. 2012, 51, 5920–5923; Angew. Chem. 2012, 124, 6022–6025;
- 12jQ. Nguyen, K. Sun, T. G. Driver, J. Am. Chem. Soc. 2012, 134, 7262–7265;
- 12kJ. G. Harrison, O. Gutierrez, N. Jana, T. G. Driver, D. J. Tantillo, J. Am. Chem. Soc. 2016, 138, 487–490;
- 12lC. Kong, N. Jana, C. Jones, T. G. Driver, J. Am. Chem. Soc. 2016, 138, 13271–13280.
- 13For leading reviews on annulations of 1,2,3-triazoles via ionic mechanism, see:
- 13aB. Chattopadhyay, V. Gevorgyan, Angew. Chem. Int. Ed. 2012, 51, 862–872; Angew. Chem. 2012, 124, 886–896;
- 13bA. V. Gulevich, V. Gevorgyan, Angew. Chem. Int. Ed. 2013, 52, 1371–1373; Angew. Chem. 2013, 125, 1411–1413;
- 13cH. M. L. Davies, J. S. Alford, Chem. Soc. Rev. 2014, 43, 5151–5162; For annulations of 1,2,3-triazoles via radical activation mechanism, see:
- 13dS. Roy, S. K. Das, B. Chattopadhyay, Angew. Chem. Int. Ed. 2018, 57, 2238–2243; Angew. Chem. 2018, 130, 2260–2265.
- 14
- 14aR. Harder, C. Wentrup, J. Am. Chem. Soc. 1976, 98, 1259–1260;
- 14bC. Wentrup, H. W. Winter, J. Am. Chem. Soc. 1980, 102, 6159–6161;
- 14cR. A. Evans, M. W. Wong, C. Wentrup, J. Am. Chem. Soc. 1996, 118, 4009–4017;
- 14dD. Kvaskoff, M. Vosswinkel, C. Wentrup, J. Am. Chem. Soc. 2011, 133, 5413–5424;
- 14eR. Huisgen, K. von Fraunberg, H. J. Sturm, Tetrahedron Lett. 1969, 10, 2589–2594;
10.1016/S0040-4039(01)88576-2 Google Scholar
- 14fR. Huisgen, K. von Fraunberg, Tetrahedron Lett. 1969, 10, 2595–2598;
10.1016/S0040-4039(01)88577-4 Google Scholar
- 14gK. von Fraunberg, R. Huisgen, Tetrahedron Lett. 1969, 10, 2599–2602.
10.1016/S0040-4039(01)88578-6 Google Scholar
- 15S. K. Das, S. Roy, H. Khatua, B. Chattopadhyay, J. Am. Chem. Soc. 2018, 140, 8429–8433.
- 16S. Roy, H. Khatua, S. K. Das, B. Chattopadhyay, Angew. Chem. Int. Ed. 2019, 58, 11439–11443; Angew. Chem. 2019, 131, 11561–11565.
- 17S. K. Das, S. Roy, H. Khatua, B. Chattopadhyay, J. Am. Chem. Soc. 2020, 142, 16211–16217.
- 18H. Khatua, S. K. Das, S. Roy, B. Chattopadhyay, Angew. Chem. Int. Ed. 2021, 60, 304–312; Angew. Chem. 2021, 133, 308–316.
- 19Y. Hu, K. Lang, C.-Q. Li, J. B. Gill, I. Kim, H.-J. Lu, K. B. Fields, M. K. Marshall, Q.-G. Cheng, X. Cui, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2019, 141, 18160–18169.
- 20H.-L. Jiang, K. Lang, H.-J. Lu, L. Wojtas, X. P. Zhang, J. Am. Chem. Soc. 2017, 139, 9164–9167.
- 21Y. Liu, C.-M. Che, Chem. Eur. J. 2010, 16, 10494–10501.
- 22J. R. Clark, K. Feng, A. Sookezian, M. C. White, Nat. Chem. 2018, 10, 583–591.
- 23U. Mueller, J. Dressel, P. Fey, R. Hanko, W. Huebsch, T. Kraemer, M. Mueller-Gliemann, M. Beuck, S. Kazda, S. Wohlfeil, A. Knorr, J. P. Stasch, S. Zaiss, From Ger. Offen. DE 4308788 A1 19940922, 1994.
- 24N. D. Paul, S. Mandal, M. Otte, X. Cui, X. P. Zhang, B. de Bruin, J. Am. Chem. Soc. 2014, 136, 1090–1096.
- 25In addition to the formation of the spirocyclic product (2 g′), we also have observed the formation of the migration product (2 g).
- 26Q. Nguyen, T. Nguyen, T. G. Driver, J. Am. Chem. Soc. 2013, 135, 620–623.
- 27C. Jones, Q. Nguyen, T. G. Driver, Angew. Chem. Int. Ed. 2014, 53, 785–788; Angew. Chem. 2014, 126, 804–807.