Heterocyclization Reagents for Rapid Assembly of N-Fused Heteroarenes from Alkenes
Huihui Zhang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorMin Wang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorDr. Xinxin Wu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chen Zhu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorHuihui Zhang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorMin Wang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorDr. Xinxin Wu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Chen Zhu
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu, 215123 China
Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032 China
Search for more papers by this authorDedicated to the 70th anniversary of the Shanghai Institute of Organic Chemistry
Graphical Abstract
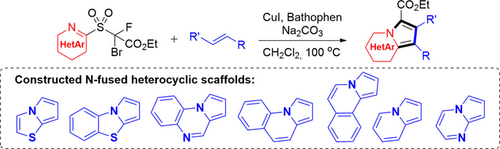
Described herein is a conceptually new approach for the synthesis of N-fused heteroarenes from alkenes. A portfolio of rationally designed heterocyclization reagents are readily prepared for the cascade reaction. A variety of N-fused heteroarenes involving seven types of heterocyclic core are accomplished.
Abstract
N-Fused heterocycles are of particular use and upmost importance in multiple fields. Herein, we disclose a conceptually new approach for the rapid assembly of N-fused heteroarenes from alkenes. A portfolio of strategically designed heterocyclization reagents are readily prepared for the cascade reaction. A plethora of N-fused heteroarenes including seven types of heterocyclic core are furnished. The protocol features a broad functional-group compatibility and high product diversity, and provides a practical tool for late-stage heteroarene elaboration.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202013089-sup-0001-misc_information.pdf12.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aJ. P. Michael, Nat. Prod. Rep. 1999, 16, 675–696;
- 1bA. A. Kalinin, V. A. Mamedov, Chem. Heterocycl. Compd. 2011, 46, 1423–1442;
- 1cL. Zhang, X. Peng, G. L. V. Damu, R. Geng, C.-H. Zhou, Med. Res. Rev. 2014, 34, 340–437;
- 1dM. D. Matveeva, R. Purgatorio, L. G. Voskressensky, C. D. Altomare, Future Med. Chem. 2019, 11, 2735–2755;
- 1eW. Cong, L. Wang, Q. Yu, J. Li, Chin. J. Org. Chem. 2018, 38, 2866–2878.
- 2N. A. McGrath, M. Brichacek, J. T. Njardarson, J. Chem. Educ. 2010, 87, 1348–1349.
- 3
- 3aM. J. Humphires, K. Matsumoto, S. L. White, K. Olden, Cancer Res. 1986, 46, 5215–5222;
- 3bM. Bols, V. H. Lillelund, H. H. Jensen, X. Liang, Chem. Rev. 2002, 102, 515–554;
- 3cW. H. Pearson, L. Guo, Tetrahedron Lett. 2001, 42, 8267–8271;
- 3dR. A. Gruters, J. J. Neefjes, M. Tersmette, R. E. Y. de Goede, A. Tulp, H. G. Huisman, F. Miedema, H. L. Ploegh, Nature 1987, 330, 74–77;
- 3eM. Facompre, C. Tardy, C. Bal-Mahieu, P. Colson, C. Perez, I. Manzanares, C. Cuevas, C. Bailly, Cancer Res. 2003, 63, 7392–7399;
- 3fC. Bailly, Curr. Med. Chem. Anti-Cancer Agents 2004, 4, 363–378.
- 4
- 4aG. Trapani, Eur. J. Med. Chem. 1994, 29, 197–204;
- 4bH. Xu, L.-L. Fan, Eur. J. Med. Chem. 2011, 46, 1919–1925;
- 4cQ. Zhang, G. Tu, Y. Zhao, T. Cheng, Tetrahedron 2002, 58, 6795–6798;
- 4dZ. Yang, C. Liu, L. Xiang, Y. Zheng, Phyther. Res. 2009, 23, 1032–1035.
- 5
- 5aG. Dannhardt, W. Kiefer, Arch. Pharm. 1994, 327, 509–514;
- 5bG. S. Singh, E. E. Mmatli, Eur. J. Med. Chem. 2011, 46, 5237–5257;
- 5cS. Hagishita, M. Yamada, K. Shirahase, T. Okada, Y. Murakami, Y. Ito, T. Matsuura, M. Wada, T. Kato, M. Ueno, Y. Chikazawa, K. Yamada, T. Ono, I. Teshirogi, I.-M. Ohtani, J. Med. Chem. 1996, 39, 3636–3658.
- 6
- 6aM. V. Reddy, M. R. Rao, D. Rhodes, M. S. Hansen, K. Rubins, F. D. Bushman, Y. Venkateswarlu, D. J. Faulkner, J. Med. Chem. 1999, 42, 1901–1907;
- 6bO. B. Østby, B. Dalhus, L.-L. Gundersen, F. Rise, A. Bast, G. R. M. M. Haenen, Eur. J. Org. Chem. 2000, 3763–3770;
- 6cA. Mertens, H. Zilch, B. König, W. Schäfer, T. Poll, W. Kampe, H. Seidel, U. Leser, H. Leinert, J. Med. Chem. 1993, 36, 2526–2535.
- 7
- 7aA. Chimirri, A. De Sarro, G. De Sarro, S. Grasso, G. R. Trimarchi, M. Zappal, J. Med. Chem. 1989, 32, 93–95;
- 7bA. Chimirri, A. De Sarro, G. De Sarro, R. Gitto, M. Zappalà, Farmaco 2001, 56, 821–826.
- 8I. Lalezari, E. L. Schwartz, J. Med. Chem. 1988, 31, 1427–1429.
- 9
- 9aS. D. Roughley, A. M. Jordan, J. Med. Chem. 2011, 54, 3451–3479;
- 9bN. Schneider, D. M. Lowe, R. A. Sayle, M. A. Tarselli, G. A. Landrum, J. Med. Chem. 2016, 59, 4385–4402;
- 9cD. G. Brown, J. Boström, J. Med. Chem. 2016, 59, 4443–4458.
- 10
- 10aL. Leontie, Synth. Met. 2005, 155, 138–145;
- 10bJ. Wan, C.-J. Zheng, M.-K. Fung, X.-K. Liu, C.-S. Lee, X.-H. Zhang, J. Mater. Chem. 2012, 22, 4502–4510;
- 10cE. Kim, M. Koh, B. J. Lim, S. B. Park, J. Am. Chem. Soc. 2011, 133, 6642–6649;
- 10dC. H. Weidner, D. H. Wadsworth, S. L. Bender, D. J. Beltman, J. Org. Chem. 1989, 54, 3660–3664;
- 10eY. R. Song, C. W. Lim, T. W. Kim, Luminescence 2016, 31, 364–371;
- 10fE. Kim, Y. Lee, S. Lee, S. B. Park, Acc. Chem. Res. 2015, 48, 538–547;
- 10gX. Zheng, R. Ji, X. Cao, Y. Ge, Anal. Chim. Acta 2017, 978, 48–54;
- 10hA. A. Kalinin, M. A. Smirnov, L. N. Islamova, G. M. Fazleeva, T. A. Vakhonina, A. I. Levitskaya, O. D. Fominykh, N. V. Ivanova, A. R. Khamatgalimov, I. R. Nizameev, Dyes Pigm. 2017, 147, 444–454;
- 10iM. K. Bayazita, K. S. Coleman, J. Am. Chem. Soc. 2009, 131, 10670–10676.
- 11For selected examples:
- 11aB. Yan, Y. Liu, Org. Lett. 2007, 9, 4323–4326;
- 11bB. Yuan, R. He, W. Shen, M. Li, Tetrahedron 2017, 73, 6092–6100;
- 11cL.-X. Wang, Y.-L. Tang, Eur. J. Org. Chem. 2017, 2207–2213;
- 11dS. U. Dighe, S. Hutait, S. Batra, ACS Comb. Sci. 2012, 14, 665–672;
- 11eK. H. Oh, S. M. Kim, S. Y. Park, J. K. Park, Org. Lett. 2016, 18, 2204–2207;
- 11fK. Xiao, C. Zhu, M. Lin, C. Song, J. Chang, ChemistrySelect 2018, 3, 11270–11272.
- 12For selected examples:
- 12aY. Tominaga, Y. Shiroshita, A. J. Hosomi, Heterocycl. Chem. 1988, 25, 1745–1749;
- 12bX. Fang, Y. Wu, J. Deng, S. Wang, Tetrahedron 2004, 60, 5487–5493;
- 12cB. Wang, W. Liu, H. Hu, Chin. J. Chem. 2006, 24, 279–281;
- 12dD. S. Allgäuer, P. Mayer, H. Mayr, J. Am. Chem. Soc. 2013, 135, 15216–15224;
- 12eF. Li, J. Chen, Y. Hou, Y. Li, X. Y. Wu, X. Tong, Org. Lett. 2015, 17, 5376–5379;
- 12fB. Shen, B. Li, B. Wang, Org. Lett. 2016, 18, 2816–2819;
- 12gY. Liu, H. Hu, J. Zhou, W. Wang, Y. He, C. Wang, Org. Biomol. Chem. 2017, 15, 5016–5024;
- 12hT. Das, P. Saha, V. K. Singh, Org. Lett. 2015, 17, 5088–5091;
- 12iH.-C. Liu, K. Liu, Z.-Y. Xue, Z.-L. He, C.-J. Wang, Org. Lett. 2015, 17, 5440–5443;
- 12jS. Muthusaravanan, S. Perumal, P. Yogeeswari, D. Sriram, Tetrahedron Lett. 2010, 51, 6439–6443.
- 13For selected examples:
- 13aJ. L. García Ruano, A. Fraile, M. R. Martín, G. Gonzalez, C. Fajardo, J. Org. Chem. 2008, 73, 8484–8490;
- 13bD. C. Wang, M. S. Xie, H. M. Guo, G. R. Qu, M. C. Zhang, S. L. You, Angew. Chem. Int. Ed. 2016, 55, 14111–14115; Angew. Chem. 2016, 128, 14317–14321;
- 13cC. R. Berry, C. A. Zificsak, A. C. Gibbs, D. J. Hlasta, Org. Lett. 2007, 9, 4099–4102;
- 13dM. Nassiri, F. J. Milani, A. Hassankhani, J. Heterocycl. Chem. 2015, 52, 1162–1166;
- 13eS. Bonte, I. O. Ghinea, R. Dinica, I. Baussanne, M. Demeunynck, Molecules 2016, 21, 332;
- 13fT. Gao, X. Chen, L. Jiang, M. Wu, H. Guo, J. Wang, S. Sun, J. Oiler, Y. Xing, Eur. J. Org. Chem. 2016, 4957–4960;
- 13gY. Shang, M. Zhang, S. Yu, K. Ju, C. Wang, X. He, Tetrahedron Lett. 2009, 50, 6981–6984.
- 14For selected examples:
- 14aA. V. Kel'in, A. W. Sromek, V. Gevorgyan, J. Am. Chem. Soc. 2001, 123, 2074–2075;
- 14bR. Martín, M. R. Rivero, S. L. Buchwald, Angew. Chem. Int. Ed. 2006, 45, 7079–7082; Angew. Chem. 2006, 118, 7237–7240;
- 14cA. R. Hardin, R. Sarpong, Org. Lett. 2007, 9, 4547–4550;
- 14dP. P. Lange, A. R. Bogdan, K. James, Adv. Synth. Catal. 2012, 354, 2373–2379;
- 14eI. V. Seregin, V. Gevorgyan, J. Am. Chem. Soc. 2006, 128, 12050–12051;
- 14fV. Helan, A. V. Gulevich, V. Gevorgyan, Chem. Sci. 2015, 6, 1928–1931;
- 14gY. Yang, T. Wu, Y. Fang, Synlett 2018, 29, 1909–1913;
- 14hY. Shi, V. Gevorgyan, Chem. Commun. 2015, 51, 17166–17169;
- 14iT. Wu, M. Chen, Y. Yang, J. Org. Chem. 2017, 82, 11304–11309;
- 14jX. Meng, P. Liao, J. Liu, X. Bi, Chem. Commun. 2014, 50, 11837–11839;
- 14kH.-Y. Zhao, Y.-C. Wang, X.-L. Cao, Q.-F. Pang, H.-S. Wang, Y.-M. Pan, RSC Adv. 2017, 7, 24011–24014;
- 14lX. Wang, X. Qiu, J. Wei, J. Liu, S. Song, W. Wang, N. Jiao, Org. Lett. 2018, 20, 2632–2636;
- 14mX. Wu, H. Xiong, S. Sun, J. Cheng, Org. Lett. 2018, 20, 1396–1399.
- 15For selected examples:
- 15aL. Zhang, X. Li, Y. Liu, D. Zhang, Chem. Commun. 2015, 51, 6633–6636;
- 15bH. Kim, K. Lee, S. Kim, P. H. Lee, Chem. Commun. 2010, 46, 6341–6343;
- 15cY. Yang, C. Xie, Y. Xie, Y. Zhang, Org. Lett. 2012, 14, 957–959;
- 15dH. Li, X. Li, Y. Yu, J. Li, Y. Liu, H. Li, W. Wang, Org. Lett. 2017, 19, 2010–2013;
- 15eD. I. Chai, M. Lautens, J. Org. Chem. 2009, 74, 3054–3061;
- 15fJ. J. Lade, B. N. Patil, P. A. Sathe, K. S. Vadagaonkar, P. Chetti, A. C. Chaskar, ChemistrySelect 2017, 2, 6811–6817;
- 15gS. Annareddygari, V. R. Kasireddy, J. Reddy, J. Heterocycl. Chem. 2019, 56, 3267–3276.
- 16For selected examples:
- 16aX. Huang, T. Zhang, Tetrahedron Lett. 2009, 50, 208–211;
- 16bY. Bai, J. Zeng, J. Ma, B. K. Gorityala, X. W. Liu, J. Comb. Chem. 2010, 12, 696–699;
- 16cF. Wang, Y. Shen, H. Hu, X. Wang, H. Wu, Y. Liu, J. Org. Chem. 2014, 79, 9556–9566;
- 16dJ. Sun, F. Wang, H. Hu, X. Wang, H. Wu, Y. Liu, J. Org. Chem. 2014, 79, 3992–3998;
- 16eM. J. Albaladejo, F. Alonso, ACS Catal. 2015, 5, 3446–3456;
- 16fH. Zhu, H. Zou, Org. Lett. 2011, 13, 2792–2794;
- 16gX. Fan, Y. Wang, Y. He, X. Zhang, Eur. J. Org. Chem. 2014, 713–717;
- 16hY. Fang, L. He, W. Pan, Y. Yang, Tetrahedron 2019, 75, 3767–3771.
- 17W. R. Pitt, D. M. Parry, B. G. Perry, C. R. Groom, J. Med. Chem. 2009, 52, 2952–2963.
- 18For selected reviews, see:
- 18aT. Pintauer, K. Matyjaszewski, Chem. Soc. Rev. 2008, 37, 1087–1097;
- 18bW. T. Eckenhoff, T. Pintauer, Catal. Rev. 2010, 52, 1–59;
- 18cM.-Y. Cao, X. Ren, Z. Lu, Tetrahedron Lett. 2015, 56, 3732–3742;
- 18dJ.-R. Chen, X.-Y. Yu, W.-J. Xiao, Synthesis 2015, 47, 604–629;
- 18eX. Wu, S. Wu, C. Zhu, Tetrahedron Lett. 2018, 59, 1328–1336.
- 19For a review:
- 19aX. Wu, C. Zhu, Acc. Chem. Res. 2020, 53, 1620–1636; For selected examples:
- 19bZ. Wu, R. Ren, C. Zhu, Angew. Chem. Int. Ed. 2016, 55, 10821–10824; Angew. Chem. 2016, 128, 10979–10982;
- 19cZ. Wu, D. Wang, Y. Liu, L. Huan, C. Zhu, J. Am. Chem. Soc. 2017, 139, 1388–1391;
- 19dY. Xu, Z. Wu, J. Jiang, Z. Ke, C. Zhu, Angew. Chem. Int. Ed. 2017, 56, 4545–4548; Angew. Chem. 2017, 129, 4616–4619;
- 19eJ. Yu, Z. Wu, C. Zhu, Angew. Chem. Int. Ed. 2018, 57, 17156–17160; Angew. Chem. 2018, 130, 17402–17406;
- 19fM. Wang, H. Zhang, J. Liu, X. Wu, C. Zhu, Angew. Chem. Int. Ed. 2019, 58, 17646–17650; Angew. Chem. 2019, 131, 17810–17814;
- 19gH. Zhang, L. Kou, D. Chen, M. Ji, X. Bao, X. Wu, C. Zhu, Org. Lett. 2020, 22, 5947–5952;
- 19hJ. Liu, S. Wu, J. Yu, C. Lu, Z. Wu, X. Wu, X. Xue, C. Zhu, Angew. Chem. Int. Ed. 2020, 59, 8195–8202; Angew. Chem. 2020, 132, 8272–8279.
- 20Deposition Number 2032457 (for 3a) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 21Deposition Number 2032458 (for 4b) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 22Deposition Number 2032459 (for 5j) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 23Deposition Number 2032460 (for 6f) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.