Low-Temperature Intramolecular [4+2] Cycloaddition of Allenes with Arenes for the Synthesis of Diene Ligands
Dr. Durga Prasad Hari
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Present address: School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS UK
These authors contributed equally to this work.
Search for more papers by this authorGuillaume Pisella
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorDr. Matthew D. Wodrich
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Search for more papers by this authorArtem V. Tsymbal
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Farzaneh Fadaei Tirani
Institute of Chemistry and Chemical Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC-GE, BCH 2111, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Rosario Scopelliti
Institute of Chemistry and Chemical Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC-GE, BCH 2111, 1015 Lausanne, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Dr. Jerome Waser
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Durga Prasad Hari
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Present address: School of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS UK
These authors contributed equally to this work.
Search for more papers by this authorGuillaume Pisella
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
These authors contributed equally to this work.
Search for more papers by this authorDr. Matthew D. Wodrich
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Search for more papers by this authorArtem V. Tsymbal
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Farzaneh Fadaei Tirani
Institute of Chemistry and Chemical Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC-GE, BCH 2111, 1015 Lausanne, Switzerland
Search for more papers by this authorDr. Rosario Scopelliti
Institute of Chemistry and Chemical Engineering, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC-GE, BCH 2111, 1015 Lausanne, Switzerland
Search for more papers by this authorCorresponding Author
Prof. Dr. Jerome Waser
Laboratory of Catalysis and Organic Synthesis, Ecole Polytechnique Fédérale de Lausanne, EPFL SB ISIC LCSO, BCH 1402, 1015 Lausanne, Switzerland
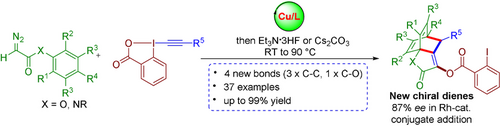
Search for more papers by this authorGraphical Abstract
A highly efficient strategy for the rapid assembly of bicyclooctadienes starting from simple diazo esters and EBX reagents through a one-pot sequential oxyalkynylation/ [4+2] allene-arene cycloaddition reaction at low temperature (23–90 °C) is reported. The obtained products are good chiral ligands for rhodium-catalyzed conjugate addition.
Abstract
The intramolecular [4+2] cycloaddition between arenes and allenes first reported by Himbert gives rapid access to rigid polycyclic scaffolds. Herein, we report a one-pot oxyalkynylation/cycloaddition reaction proceeding under mild conditions (23–90 °C) and providing complex polycyclic architectures with high efficiency, and atom and step economy. The bicyclo[2.2.2]octadiene products were obtained with a wide variety of useful functional groups and were successfully applied as chiral ligands for metal catalysis. Computational studies gave a first rationalization of the low activation energy for the cycloaddition based on counter-intuitive favorable dispersive interactions in the transition state.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202012299-sup-0001-misc_information.pdf15.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. Rodriguez, D. Bonne, Stereoselective Multiple Bond-Forming Transformations in Organic Synthesis, Wiley, Hoboken, 2015.
10.1002/9781119006220 Google Scholar
- 2
- 2aW. Carruthers, Cycloaddition Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 1990;
- 2bS. Kobayashi, K. A. Jørgensen, Eds., Cycloaddition Reactions in Organic Synthesis, Wiley-VCH, Weinheim, 2001;
10.1002/3527600256 Google Scholar
- 2cN. Nishiwaki, Methods and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, Hoboken, 2014.
10.1002/9781118778173 Google Scholar
- 3
- 3aG. Brieger, J. N. Bennett, Chem. Rev. 1980, 80, 63;
- 3bK. C. Nicolaou, S. A. Snyder, T. Montagnon, G. Vassilikogiannakis, Angew. Chem. Int. Ed. 2002, 41, 1668;
10.1002/1521-3773(20020517)41:10<1668::AID-ANIE1668>3.0.CO;2-Z CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 1742.
- 4K. Takao, R. Munakata, K. Tadano, Chem. Rev. 2005, 105, 4779.
- 5
- 5aN. Zydziak, B. Yameen, C. Barner-Kowollik, Polym. Chem. 2013, 4, 4072;
- 5bW. Binder, C. Kluger, Curr. Org. Chem. 2006, 10, 1791.
- 6L. C. Bouchez, M. Rusch, M.-H. Larraufie, Curr. Org. Chem. 2016, 20, 2358.
- 7F. Fringuelli, A. Taticchi, The Diels—Alder Reaction: Selected Practical Methods, Wiley, Chichester, 2002.
- 8D. W. Thomas, H. Achenbach, K. Biemann, J. Am. Chem. Soc. 1966, 88, 3423.
- 9M. Connock, A. Juarez-Garcia, S. Jowett, E. Frew, Z. Liu, R. Taylor, A. Fry-Smith, E. Day, N. Lintzeris, T. Roberts, A. Burls, R. S. Taylor, Health Technology Assessment 2007, 11, 1–171.
- 10
- 10aC. Fischer, C. Defieber, T. Suzuki, E. M. Carreira, J. Am. Chem. Soc. 2004, 126, 1628;
- 10bC. Defieber, H. Grutzmacher, E. M. Carreira, Angew. Chem. Int. Ed. 2008, 47, 4482; Angew. Chem. 2008, 120, 4558;
- 10cN. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2004, 126, 13584;
- 10dY. Otomaru, K. Okamoto, R. Shintani, T. Hayashi, J. Org. Chem. 2005, 70, 2503;
- 10eK. Okamoto, T. Hayashi, V. H. Rawal, Chem. Commun. 2009, 4815.
- 11Selected examples:
- 11aN. Kumar, M. Kiuchi, J. A. Tallarico, S. L. Schreiber, Org. Lett. 2005, 7, 2535;
- 11bM. Dai, D. Sarlah, M. Yu, S. J. Danishefsky, G. O. Jones, K. N. Houk, J. Am. Chem. Soc. 2007, 129, 645;
- 11cK. Ishihara, M. Fushimi, J. Am. Chem. Soc. 2008, 130, 7532;
- 11dJ.-P. Krieger, G. Ricci, D. Lesuisse, C. Meyer, J. Cossy, Angew. Chem. Int. Ed. 2014, 53, 8705; Angew. Chem. 2014, 126, 8849;
- 11eK. B. Hamal, R. Bam, W. A. Chalifoux, Synlett 2016, 27, 2161;
- 11fM. Hatano, T. Sakamoto, T. Mizuno, Y. Goto, K. Ishihara, J. Am. Chem. Soc. 2018, 140, 16253.
- 12F. Fringuelli, A. Taticchi, Dienes in the Diels–Alder Reaction, Wiley, New York, 1990.
- 13G. Himbert, L. Henn, Angew. Chem. Int. Ed. Engl. 1982, 21, 620; Angew. Chem. 1982, 94, 631.
- 14
- 14aG. Himbert, D. Fink, Tetrahedron Lett. 1985, 26, 4363;
- 14bG. Himbert, D. Fink, M. Stürm, Z. Naturforsch. B 1994, 49, 63.
- 15
- 15aL. Henn, G. Himbert, K. Diehl, M. Kaftory, Chem. Ber. 1986, 119, 1953;
- 15bG. Himbert, K. Diehl, H.-J. Schlindwein, Chem. Ber. 1986, 119, 3227;
- 15cK. Diehl, G. Himbert, Chem. Ber. 1986, 119, 3812;
- 15dH.-J. Schlindwein, K. Diehl, G. Himbert, Chem. Ber. 1989, 122, 577;
- 15eG. Himbert, H.-J. Schlindwein, Z. Naturforsch. B 1992, 47, 1785;
- 15fG. Himbert, D. Fink, J. Org. Chem. 1996, 338, 355.
- 16G. Himbert, D. Fink, J. Prakt. Chem. 1994, 336, 654.
- 17
- 17aL. S. Trifonov, A. S. Orahovats, Helv. Chim. Acta 1986, 69, 1585;
- 17bL. S. Trifonov, A. S. Orahovats, Helv. Chim. Acta 1987, 70, 1732;
- 17cL. S. Trifonov, A. S. Orahovats, Helv. Chim. Acta 1987, 70, 262;
- 17dL. S. Trifonov, A. S. Orahovats, Helv. Chim. Acta 1989, 72, 59.
- 18G. Himbert, M. Ruppmich, H. Knöringer, J. Chin. Chem. Soc. 2003, 50, 143.
- 19L. S. Trifonov, S. D. Simova, A. S. Orahovats, Tetrahedron Lett. 1987, 28, 3391.
- 20Y. Schmidt, J. K. Lam, H. V. Pham, K. N. Houk, C. D. Vanderwal, J. Am. Chem. Soc. 2013, 135, 7339.
- 21G. Cheng, X. He, L. Tian, J. Chen, C. Li, X. Jia, J. Li, J. Org. Chem. 2015, 80, 11100.
- 22For the only example of application of the Himbert reaction in total synthesis, see: J. K. Lam, Y. Schmidt, C. D. Vanderwal, Org. Lett. 2012, 14, 5566.
- 23X. Mo, B. Chen, G. Zhang, Angew. Chem. Int. Ed. 2020, 59, 13997; Angew. Chem. 2020, 132, 14101.
- 24U. Streit, F. Birbaum, A. Quattropani, C. G. Bochet, J. Org. Chem. 2013, 78, 6890.
- 25
- 25aJ. Waser, Top. Curr. Chem. 2015, 373, 187;
- 25bY. Li, D. P. Hari, M. V. Vita, J. Waser, Angew. Chem. Int. Ed. 2016, 55, 4436; Angew. Chem. 2016, 128, 4512.
- 26
- 26aD. P. Hari, J. Waser, J. Am. Chem. Soc. 2016, 138, 2190;
- 26bD. P. Hari, J. Waser, J. Am. Chem. Soc. 2017, 139, 8420.
- 27There are no reports of carboxy-substituted allene esters and only rare reports of alkoxy substituted allene esters:
- 27aM. J. Sleeman, G. V. Meehan, Tetrahedron Lett. 1989, 30, 3345;
- 27bY. Nagao, K. Kim, S. Sano, H. Kakegawa, W. S. Lee, H. Shimizu, M. Shiro, N. Katunuma, Tetrahedron Lett. 1996, 37, 861; Simple alkoxy-substituted allenes have been more often used:
- 27cR. Zimmer, H.-U. Reissig, Angew. Chem. Int. Ed. Engl. 1988, 27, 1518; Angew. Chem. 1988, 100, 1576;
- 27dV. M. Schmiedel, H. U. Reissig, Curr. Org. Chem. 2019, 23, 2976.
- 28Deposition Number 1848760 (for 6a) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 29The structure of the diastereoisomers was assigned in analogy to the work in ref. [20] and [22].
- 30In the case of less reactive substituted alkynes, the presence of the tert-butyl groups was necessary for the success of the cycloaddition.
- 31See Supporting Information for details on the optimization of the reaction conditions.
- 32Deposition Number 1848773 (for 6r) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 33The reaction with alkyl-EBXs required 50 °C to form allenes, which undergo spontaneous cyclization to give the corresponding Himbert products.
- 34Enantionenriched 5 r was obtained following our previously published methodology, ref [26b].
- 35F. M. Bickelhaupt, K. N. Houk, Angew. Chem. Int. Ed. 2017, 56, 10070; Angew. Chem. 2017, 129, 10204, and references therein.
- 36Deposition Number 1850113 (for 15) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 37Deposition Number 1945514 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 38Deposition Number 2027174 (for 20b) contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 39T. Nishimura, Y. Ichikawa, T. Hayashi, N. Onishi, M. Shiotsuki, T. Masuda, Organometallics 2009, 28, 4890.
- 40See Figure S1 in Supporting Information for an overlay of the structures of 20 b and 20 c.