(Aza)Acenes Share the C2 Bridge with (Anti)Aromatic Macrocycles: Local vs. Global Delocalization Paths
Krzysztof Bartkowski
Department of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50383 Wrocław, Poland
Search for more papers by this authorCorresponding Author
Prof. Miłosz Pawlicki
Department of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50383 Wrocław, Poland
Faculty of Chemistry, Jagiellonian University, Gronostajowa 2, 30387 Kraków, Poland
Search for more papers by this authorKrzysztof Bartkowski
Department of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50383 Wrocław, Poland
Search for more papers by this authorCorresponding Author
Prof. Miłosz Pawlicki
Department of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50383 Wrocław, Poland
Faculty of Chemistry, Jagiellonian University, Gronostajowa 2, 30387 Kraków, Poland
Search for more papers by this authorDedicated to Professor Lechosław Latos-Grażyński on the occasion of his 70th birthday
Graphical Abstract
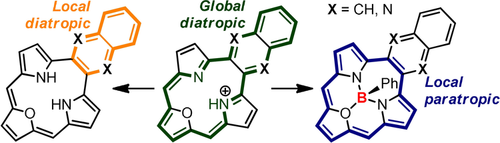
Global switch for local effect: The linear fusion of (aza)acene and a redox switchable macrocycle (i.e. triphyrin(2.1.1)) gives a set of fused systems merging two π-conjugated clouds. A competition between local and global effects can be distinguished as documented in spectroscopic properties with a domination of (aza)acene diatropic current obtained after a redox switching within the macrocyclic flank.
Abstract
A strong conjugation present in fused systems plays a crucial role in tuning of the properties that would be showing a dependence on the efficiency of π-electrons coupling. The π-cloud available in the final structure can be drastically influenced by a side- or a linear fusion of unsaturated and conjugated hydrocarbons. The linear welding of naphthalene/anthracene or quinoxaline/benzo[g]quinoxaline with triphyrin(2.1.1) gives structures where the competition between local and global delocalization is distinguished. The aromatic character observed in skeletons strongly depends on the oxidation state of the macrocyclic flanking and is either extended over the whole system or kept as a composition of local currents (diatropic and paratropic) of incorporated units. The hybrid systems show the properties derived from the π-conjugations that interlace one another but also show a significant independence of (aza)acene subunits reflected in the observed spectroscopic properties.
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202011848-sup-0001-misc_information.pdf6.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Gleiter, G. Haberhauer, Aromaticity and Other Conjugation Effects, Wiley-VCH, Weinheim, 2012;
- 1bT. M. Krygowski, M. K. Cyrański, Z. Czarnocki, G. Häfelinger, A. R. Katritzky, Tetrahedron 2000, 56, 1783–1796.
- 2J. A. N. F. Gomes, R. B. Mallion, Chem. Rev. 2001, 101, 1349–1383.
- 3
- 3aR. Dorel, A. M. Echavarren, Eur. J. Org. Chem. 2017, 14–24;
- 3bA. Bedi, O. Gidron, Acc. Chem. Res. 2019, 52, 2482–2490;
- 3cU. H. F. Bunz, Acc. Chem. Res. 2015, 48, 1676–1686;
- 3dA. Mateo-Alonso, Chem. Soc. Rev. 2014, 43, 6311–6324;
- 3eJ. E. Anthony, Angew. Chem. Int. Ed. 2008, 47, 452–483; Angew. Chem. 2008, 120, 460–492;
- 3fU. H. F. Bunz, J. Freudenberg, Acc. Chem. Res. 2019, 52, 1575–1587;
- 3gX. Shi, C. Chi, Chem. Rec. 2016, 16, 1690–1700.
- 4
- 4aK. J. Thorley, J. E. Anthony, Isr. J. Chem. 2014, 54, 642–649;
- 4bC. H. Suresh, S. R. Gadre, J. Org. Chem. 1999, 64, 2505–2512;
- 4cM. Solà, Front. Chem. 2013, 1, 22;
- 4dM. Bendikov, H. M. Duong, K. Starkey, K. N. Houk, E. A. Carter, F. Wudl, J. Am. Chem. Soc. 2004, 126, 7416–7417.
- 5T. Tanaka, A. Osuka, Chem. Soc. Rev. 2015, 44, 943–969.
- 6
- 6aS. Richeter, C. Jeandon, J.-P. Gisselbrecht, R. Ruppert, H. J. Callot, J. Am. Chem. Soc. 2002, 124, 6168–6179;
- 6bS. Fox, R. W. Boyle, Chem. Commun. 2004, 1322–1323;
- 6cD.-M. Shen, C. Liu, Q.-Y. Chen, Chem. Commun. 2005, 4982–4984;
- 6dE. Hao, F. R. Fronczek, M. G. H. Vincente, J. Org. Chem. 2006, 71, 1233–1236;
- 6eH. S. Gill, M. Marmjanz, J. Santamaria, I. Finger, M. J. Scott, Angew. Chem. Int. Ed. 2004, 43, 485–490; Angew. Chem. 2004, 116, 491–496;
- 6fA. N. Cammidge, P. J. Scaife, G. Berber, D. L. Hughes, Org. Lett. 2005, 7, 3413–3416;
- 6gM. Tanaka, S. Hayashi, S. Eu, T. Umeyama, Y. Matano, H. Imahori, Chem. Commun. 2007, 2069–2071;
- 6hO. Yamane, K. Sugiura, H. Miyasaka, K. Nakamura, T. Fujimoto, K. Nakamura, T. Kaneda, Y. Sakata, M. Yamashita, Chem. Lett. 2004, 33, 40–41;
- 6iK. Kurotobi, K. S. Kim, S. B. Noh, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2006, 45, 3944–3947; Angew. Chem. 2006, 118, 4048–4051;
- 6jN. K. S. Davis, M. Pawlicki, H. L. Anderson, Org. Lett. 2008, 10, 3945–3947.
- 7M. Pawlicki, Synlett 2020, 31, 1–6.
- 8
- 8aW. Stawski, K. Hurej, J. Skonieczny, M. Pawlicki, Angew. Chem. Int. Ed. 2019, 58, 10946–10950; Angew. Chem. 2019, 131, 11062–11066;
- 8bM. Pawlicki, K. Hurej, K. Kwiecińska, L. Szterenberg, L. Latos-Grażyński, Chem. Commun. 2015, 51, 11362–11365;
- 8cK. Hurej, W. Stawski, L. Latos-Grażyński, M. Pawlicki, Chem. Asian J. 2016, 11, 3329–3333.
- 9M. Farinone, J. Cybińska, M. Pawlicki, Org. Chem. Front. 2019, 6, 2825–2832.
- 10R. Breslow, Acc. Chem. Res. 1973, 6, 393–398.
- 11
- 11aM. D. Peeks, T. D. W. Claridge, H. L. Anderson, Nature 2017, 541, 200–203;
- 11bM. D. Peeks, M. Jirasek, T. D. W. Claridge, H. L. Anderson, Angew. Chem. Int. Ed. 2019, 58, 15717–15720; Angew. Chem. 2019, 131, 15864–15867;
- 11cY. Ni, T. Y. Gopalakrishna, H. Phan, T. Kim, T. S. Herng, Y. Han, T. Tao, J. Ding, D. Kim, J. Wu, Nat. Chem. 2020, 12, 242–248;
- 11dZ. Li, T. Y. Gopalakrishna, Y. Han, Y. Gu, L. Yuan, W. Zeng, D. Casanova, J. Wu, J. Am. Chem. Soc. 2019, 141, 16266–16270;
- 11eL. Ren, T. Y. Gopalakrishna, I.-H. Park, Y. Han, J. Wu, Angew. Chem. Int. Ed. 2020, 59, 2230–2234; Angew. Chem. 2020, 132, 2250–2254.
- 12
- 12aM. J. Crossley, P. L. Burn, J. Chem. Soc. Chem. Commun. 1987, 39–40;
- 12bM. J. Crossley, L. G. King, J. Chem. Soc. Chem. Commun. 1984, 920–922;
- 12cT. Khoury, M. J. Crossley, Chem. Commun. 2007, 4851–4853;
- 12dM. J. Crossley, P. L. Burn, S. S. Chew, F. B. Cuttance, I. A. Newsom, J. Chem. Soc. Chem. Commun. 1991, 1564–1566;
- 12eK. Kise, A. Osuka, Chem. Eur. J. 2019, 25, 15493–15497;
- 12fD. Kuzuhara, M. Sakaguchi, W. Furukawa, T. Okabe, N. Aratani, H. Yamada, Molecules 2017, 22, 908;
- 12gB. Szyszko, D. Dróżdż, A. Sarwa, S. G. Mucha, A. Białońska, M. J. Białek, K. Matczyszyn, L. Latos-Grażyński, Org. Chem. Front. 2020, 7, 1430–1436.
- 13
- 13aH. Uoyama, K. S. Kim, K. Kuroki, J.-Y. Shin, T. Nagata, T. Okujima, H. Yamada, N. Ono, D. Kim, H. Uno, Chem. Eur. J. 2010, 16, 4063–4074;
- 13bJ. Nakamura, T. Okujima, Y. Tomimori, N. Komobuchi, H. Yamada, H. Uno, N. Ono, Heterocycles 2010, 80, 1165–1175;
- 13cM. Pawlicki, M. Morisue, N. K. S. Davis, D. G. McLean, J. H. Haley, E. Beuerman, M. Drobizhev, A. Rebane, A. L. Thompson, S. I. Pascu, G. Accorsi, N. Armaroli, H. L. Anderson, Chem. Sci. 2012, 3, 1541–1547;
- 13dK. Oohora, A. Ogawa, T. Fukuda, A. Onoda, J. Hasegawa, T. Hayashi, Angew. Chem. Int. Ed. 2015, 54, 6227–6230; Angew. Chem. 2015, 127, 6325–6328.
- 14
- 14aZ.-L. Xue, Z. Shen, J. Mack, D. Kuzuhara, H. Yamada, T. Okujima, N. Ono, X.-Z. You, N. Kobayashi, J. Am. Chem. Soc. 2008, 130, 16478–16479;
- 14bK. S. Anju, S. Ramakrishnan, A. Srinivasan, Org. Lett. 2011, 13, 2498–2501;
- 14cD. Kuzuhara, Y. Sakakibara, S. Mori, T. Okujima, H. Uno, H. Yamada, Angew. Chem. Int. Ed. 2013, 52, 3360–3363; Angew. Chem. 2013, 125, 3444–3447;
- 14dD. Kuzuhara, S. Kawatsu, W. Furukawa, H. Hayashi, N. Aratani, H. Yamada, Eur. J. Org. Chem. 2018, 2122–2129;
- 14eA. Kumar, K. Thorat, M. Ravikanth, Org. Lett. 2018, 20, 4871–4874;
- 14fK. N. Panda, K. G. Thorat, M. Ravikanth, J. Org. Chem. 2018, 83, 12945–12950;
- 14gD. Kuzuhara, H. Yamada, Heterocycles 2013, 87, 1209–1240.
- 15
- 15aM. Pawlicki, K. Hurej, L. Szterenberg, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2014, 53, 2992–2996; Angew. Chem. 2014, 126, 3036–3040;
- 15bM. Pawlicki, M. Garbicz, L. Szterenberg, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2015, 54, 1906–1909; Angew. Chem. 2015, 127, 1926–1929;
- 15cK. Bartkowski, M. Dimitrova, P. J. Chmielewski, D. Sundholm, M. Pawlicki, Chem. Eur. J. 2019, 25, 15477–15482.
- 16
- 16aD. Bailey, V. E. Williams, Tetrahedron Lett. 2004, 45, 2511;
- 16bT. Iwanaga, N. Asano, H. Yamada, S. Toyota, Tetrahedron Lett. 2019, 60, 1113–1116.
- 17C. B. Black, B. Andrioletti, A. C. Try, C. Ruiperez, J. L. Sessler, J. Am. Chem. Soc. 1999, 121, 10438–10439.
- 18S. Cho, Z. S. Yoon, K. S. Kim, M.-C. Yoon, D.-G. Cho, J. L. Sessler, D. Kim, J. Phys. Chem. Lett. 2010, 1, 895–900.
- 19B. Basumatary, R. V. R. Reddy, J. Sankar, Angew. Chem. Int. Ed. 2018, 57, 5052–5056; Angew. Chem. 2018, 130, 5146–5150.
- 20M. Pawlicki, L. Latos-Grażyński, Chem. Asian J. 2015, 10, 1438–1451.
- 21
- 21aM. Pawlicki, L. Szterenberg, L. Latos-Grażyński, J. Org. Chem. 2002, 67, 5644–5653;
- 21bJ. Klajn, W. Stawski, J. Cybińska, P. J. Chmielewski, M. Pawlicki, Chem. Commun. 2019, 55, 4558–4561.
- 22
- 22aI. C. Calder, F. Sondheimer, Chem. Commun. 1966, 904–905;
- 22bE. L. Spitler, C. A. Johnson II, M. M. Haley, Chem. Rev. 2006, 106, 5344–5386;
- 22cC. D. Stevenson, T. L. Kurth, J. Am. Chem. Soc. 2000, 122, 722–723.
- 23
- 23aJ. A. Cissell, T. P. Vaid, G. P. A. Yap, J. Am. Chem. Soc. 2007, 129, 7841–7847;
- 23bJ. A. Cissell, T. P. Vaid, A. G. DiPasquale, A. L. Rheingold, Inorg. Chem. 2007, 46, 7713–7715;
- 23cA. Młodzianowska, L. Latos-Grażyński, L. Szterenberg, M. Stępień, Inorg. Chem. 2007, 46, 6950–6957;
- 23dA. Idec, M. Pawlicki, L. Latos-Grażyński, Inorg. Chem. 2017, 56, 10337–10352;
- 23eM. Pawlicki, A. Kędzia, D. Bykowski, L. Latos-Grażyński, Chem. Eur. J. 2014, 20, 17500–17506.
- 24T. Kakui, S. Sugawara, Y. Hirata, S. Kojima, Y. Yamamoto, Chem. Eur. J. 2011, 17, 7768–7771.
- 25
- 25aC. K. Frederickson, B. D. Rose, M. M. Haley, Acc. Chem. Res. 2017, 50, 977–987;
- 25bJ. L. Marshall, K. Uchida, C. K. Frederockson, C. Schutt, A. M. Zeidell, K. P. Goetz, T. W. Finn, K. Jarolimek, L. N. Zakharov, C. Risko, R. Herges, O. D. Jurchescu, M. M. Haley, Chem. Sci. 2016, 7, 5547–5558;
- 25cR. E. Messersmith, S. Yadav, M. A. Siegler, H. Ottosson, J. D. Tovar, J. Org. Chem. 2017, 82, 13440–13448.
- 26Deposition Numbers 2020640 (for 7a) 2020639 (for 9a), 2020638 (for 6), 2020637 (for 9b) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 27Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta, P. von R. Schleyer, Chem. Rev. 2005, 105, 3842–3888.
- 28
- 28aR. Herges, D. Geuenich, J. Phys. Chem. A 2001, 105, 3214–3220;
- 28bD. Geuenich, K. Hess, F. Köhler, R. Herges, Chem. Rev. 2005, 105, 3758.