Stereoselective Access to Fully Substituted Aldehyde-Derived Silyl Enol Ethers by Iridium-Catalyzed Alkene Isomerization
Itai Massad
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorDr. Heiko Sommer
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorCorresponding Author
Prof. Dr. Ilan Marek
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorItai Massad
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorDr. Heiko Sommer
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorCorresponding Author
Prof. Dr. Ilan Marek
Schulich Faculty of Chemistry, Technion—Israel Institute of Technology, Technion City, Haifa, 3200009 Israel
Search for more papers by this authorDedicated to Professor Moris Eisen on the occasion of his 60th birthday
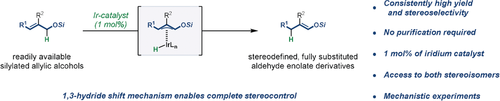
Graphical Abstract
Zig-zag forever: An Ir-based alkene isomerization catalyst generates fully substituted aldehyde enolate derivatives with complete stereocontrol, starting from simple silylated allylic alcohols. The stereo- and regioselectivity of isomerization are dictated by the preference towards zig-zig-shaped allyliridium hydride intermediates, enabling selective access to either stereoisomer of a given enolate.
Abstract
An in situ generated cationic Ir-catalyst isomerizes simple allylic silyl ethers into valuable, fully substituted aldehyde-derived silyl enol ethers. Importantly, by judicious choice of substrate, either of the two possible stereoisomers of a given enolate derivative is accessible with complete stereoselectivity. One-pot isomerization-aldol and isomerization-allylation processes illustrate the synthetic utility of this method.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie202005058-sup-0001-misc_information.pdf5.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Modern Carbonyl Chemistry (Ed.: ), Wiley-VCH, Weinheim, 2000.
- 2
- 2aW. A. Kleschick, C. T. Buse, C. H. Heathcock, J. Am. Chem. Soc. 1977, 99, 247;
- 2bC. H. Heathcock, J. Lampe, J. Org. Chem. 1983, 48, 4330;
- 2cE. M. Carreira, L. Kvaerno, Classics in Stereoselective Synthesis, Wiley-VCH, Weinheim, 2009.
- 3
- 3aR. E. Ireland, A. K. Willard, Tetrahedron Lett. 1975, 16, 3975;
10.1016/S0040-4039(00)91213-9 Google Scholar
- 3bR. E. Ireland, P. Wipf, J. D. Armstrong III, J. Org. Chem. 1991, 56, 650.
- 4Review: Y. Minko, I. Marek, Chem. Commun. 2014, 50, 12597.
- 5
- 5aS. Hosokawa, K. Sekiguchi, M. Enemoto, S. Kobayashi, Tetrahedron Lett. 2000, 41, 6429;
- 5bJ. M. Manthorpe, J. L. Gleason, J. Am. Chem. Soc. 2001, 123, 2091;
- 5cJ. M. Manthorpe, J. L. Gleason, Angew. Chem. Int. Ed. 2002, 41, 2338;
10.1002/1521-3773(20020703)41:13<2338::AID-ANIE2338>3.0.CO;2-M CAS PubMed Web of Science® Google ScholarAngew. Chem. 2002, 114, 2444;
- 5dT. Abe, T. Suzuki, K. Sekiguchi, S. Hosokawa, S. Kobayashi, Tetrahedron Lett. 2003, 44, 9303;
- 5eE. D. Burke, J. L. Gleason, Org. Lett. 2004, 6, 405;
- 5fA. Arpin, J. M. Manthorpe, J. L. Gleason, Org. Lett. 2006, 8, 1359;
- 5gD. A. Kummer, W. J. Chain, M. R. Morales, O. Quiroga, A. G. Myers, J. Am. Chem. Soc. 2008, 130, 13231;
- 5hE. A. Tiong, J. L. Gleason, Org. Lett. 2009, 11, 1725;
- 5iM. R. Morales, K. T. Mellem, A. G. Myers, Angew. Chem. Int. Ed. 2012, 51, 4568; Angew. Chem. 2012, 124, 4646;
- 5jJ. W. Medley, M. Movassaghi, Angew. Chem. Int. Ed. 2012, 51, 4572; Angew. Chem. 2012, 124, 4650;
- 5kY. Minko, M. Pasco, L. Lercher, M. Botoshansky, I. Marek, Nature 2012, 490, 522;
- 5lY. Minko, M. Pasco, L. Lercher, M. Botoshansky, I. Marek, Nat. Protoc. 2013, 8, 749;
- 5mE. A. Tiong, D. Rivalti, B. M. Williams, J. L. Gleason, Angew. Chem. Int. Ed. 2013, 52, 3442; Angew. Chem. 2013, 125, 3526;
- 5nC. Allais, A. S. Tsai, P. Nuhant, W. R. Roush, Angew. Chem. Int. Ed. 2013, 52, 12888; Angew. Chem. 2013, 125, 13126;
- 5oI. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik, J. P. Das, J. Am. Chem. Soc. 2014, 136, 2682;
- 5pP. Starkov, J. T. Moore, D. C. Duquette, B. M. Stoltz, I. Marek, J. Am. Chem. Soc. 2017, 139, 9615;
- 5qZ. Nairoukh, I. Marek, Angew. Chem. Int. Ed. 2015, 54, 14393; Angew. Chem. 2015, 127, 14601.
- 6
- 6aY. C. Qin, C. E. Stivala, A. Zakarian, Angew. Chem. Int. Ed. 2007, 46, 7466; Angew. Chem. 2007, 119, 7610;
- 6bC. E. Stivala, A. Zakarian, J. Am. Chem. Soc. 2008, 130, 3774;
- 6cC. E. Stivala, A. Zakarian, Org. Lett. 2009, 11, 839;
- 6dZ. Gu, A. T. Herrmann, C. E. Stivala, A. Zakarian, Synlett 2010, 1717.
- 7
- 7aR. Haener, T. Laube, D. Seebach, J. Am. Chem. Soc. 1985, 107, 5396;
- 7bD. Seebach, R. Amstutz, T. Laube, W. B. Schweizer, J. D. Dunitz, J. Am. Chem. Soc. 1985, 107, 5403;
- 7cA. Tsubouchi, K. Onishi, T. Takeda, J. Am. Chem. Soc. 2006, 128, 14268;
- 7dI. Kuwajima, M. Kato, J. Chem. Soc. Chem. Commun. 1979, 708;
- 7eI. Kuwajima, M. Kato, A. Mori, Tetrahedron Lett. 1980, 21, 2745;
- 7fM. Kato, A. Mori, H. Oshino, J. Enda, K. Kobayashi, I. Kuwajima, J. Am. Chem. Soc. 1984, 106, 1773;
- 7gA. Tsubouchi, S. Enatsu, R. Kanno, T. Takeda, Angew. Chem. Int. Ed. 2010, 49, 7089; Angew. Chem. 2010, 122, 7243;
- 7hE. Haimov, Z. Nairoukh, A. Shterenberg, T. Berkovitz, T. F. Jamison, I. Marek, Angew. Chem. Int. Ed. 2016, 55, 5517; Angew. Chem. 2016, 128, 5607.
- 8Y. Horiguchi, S. Matsuzawa, E. Nakamura, I. Kuwajima, Tetrahedron Lett. 1986, 27, 4025.
- 9D. Zhang, J. M. Ready, Org. Lett. 2005, 7, 5681.
- 10P.-Y. Wang, G. Duret, I. Marek, Angew. Chem. Int. Ed. 2019, 58, 14995; Angew. Chem. 2019, 131, 15137.
- 11Reviews:
- 11aA. Vasseur, J. Bruffaerts, I. Marek, Nat. Chem. 2016, 8, 209;
- 11bH. Sommer, F. Julia-Hernandez, R. Martin, I. Marek, ACS Cent. Sci. 2018, 4, 153;
- 11cJ. J. Molloy, T. Morack, R. Gilmour, Angew. Chem. Int. Ed. 2019, 58, 13654; Angew. Chem. 2019, 131, 13789;
- 11dI. Massad, I. Marek, ACS Catal. 2020, 10, 5793.
- 12Review: N. Ahlsten, A. Bartoszewicz, B. Martín-Matute, Dalton Trans. 2012, 41, 1660.
- 131,2-hydride shift mechanism:
- 13aM. Krel, J.-Y. Lallemand, C. Guillou, Synlett 2005, 2043;
- 13bY. C. Kavanagh, M. Chaney, J. Muldoon, P. Evans, J. Org. Chem. 2008, 73, 8601;
- 13cL. Mantilli, C. Mazet, Chimia 2009, 63, 35;
- 13dL. Mantilli, C. Mazet, Tetrahedron Lett. 2009, 50, 4141;
- 13eL. Mantilli, D. Gérard, S. Torche, C. Besnard, C. Mazet, Angew. Chem. Int. Ed. 2009, 48, 5143; Angew. Chem. 2009, 121, 5245;
- 13fL. Mantilli, C. Mazet, Chem. Commun. 2010, 46, 445;
- 13gL. Mantilli, D. Gérard, S. Torche, C. Besnard, C. Mazet, Pure. Appl. Chem. 2010, 82, 1461;
- 13hL. Mantilli, D. Gérard, S. Torche, C. Besnard, C. Mazet, Chem. Eur. J. 2010, 16, 12736;
- 13iA. Quintard, A. Alexakis, C. Mazet, Angew. Chem. Int. Ed. 2011, 50, 2354; Angew. Chem. 2011, 123, 2402;
- 13jL. Mantilli, D. Gérard, C. Besnard, C. Mazet, Eur. J. Inorg. Chem. 2012, 3320;
- 13kH. Li, C. Mazet, Org. Lett. 2013, 15, 6170;
- 13lH. Li, C. Mazet, J. Am. Chem. Soc. 2015, 137, 10720;
- 13mH. Li, C. Mazet, Acc. Chem. Res. 2016, 49, 1232;
- 13nC. Romano, C. Mazet, J. Am. Chem. Soc. 2018, 140, 4743.
- 14For 1,3-hydride shift mechanism, see:
- 14aD. Baudry, M. Ephritikhine, H. Felkin, J. Chem. Soc. Chem. Commun. 1978, 694;
- 14bJ. J. Oltvoort, C. A. A. Van Boeckel, J. H. de Koning, J. H. van Boom, Synthesis 1981, 305;
- 14cT. Moriya, A. Suzuki, N. Miyaura, Tetrahedron Lett. 1995, 36, 1887;
- 14dT. Ohmura, Y. Shirai, Y. Yamamoto, N. Miyaura, Chem. Commun. 1998, 1337;
- 14eT. Ohmura, Y. Yamamoto, N. Miyaura, Organometallics 1999, 18, 413;
- 14fY. Yamamoto, T. Miyairi, T. Ohmura, N. Miyaura, J. Org. Chem. 1999, 64, 296;
- 14gS. G. Nelson, C. J. Bungard, K. Wang, J. Am. Chem. Soc. 2003, 125, 13000;
- 14hS. G. Nelson, K. Wang, J. Am. Chem. Soc. 2006, 128, 4232;
- 14iB. D. Stevens, C. J. Bungard, S. G. Nelson, J. Org. Chem. 2006, 71, 6397;
- 14jK. Wang, C. J. Bungard, S. G. Nelson, Org. Lett. 2007, 9, 2325;
- 14kH. J. Lim, C. R. Smith, T. V. RajanBabu, J. Org. Chem. 2009, 74, 4565;
- 14lM. G. McLaughlin, M. J. Cook, J. Org. Chem. 2012, 77, 2058;
- 14mS. Biswas, Z. Huang, Y. Choliy, D. Y. Wang, M. Brookhart, K. Krogh-Jespersen, A. S. Goldman, J. Am. Chem. Soc. 2012, 134, 13276;
- 14nT. Miura, Y. Nishida, M. Morimoto, M. Murakami, J. Am. Chem. Soc. 2013, 135, 11497;
- 14oL. Lin, K. Yamamoto, H. Mitsunuma, Y. Kanzaki, S. Matsunaga, M. Kanai, J. Am. Chem. Soc. 2015, 137, 15418.
- 15H. Sommer, T. Weissbrod, I. Marek, ACS Catal. 2019, 9, 2400.
- 16Silyl enol ether stereochemistry was determined by NOESY experiments.
- 17J. Feng, M. Holmes, M. J. Krische, Chem. Rev. 2017, 117, 12564.
- 18E. Larionov, L. Lin, L. Guénée, C. Mazet, J. Am. Chem. Soc. 2014, 136, 16882.
- 19L. Lin, C. Romano, C. Mazet, J. Am. Chem. Soc. 2016, 138, 10344.
- 20Y. Yamasaki, T. Kumagai, S. Kanno, F. Kakiuchi, T. Kochi, J. Org. Chem. 2018, 83, 9322.
- 21The relative stereochemistry of 5 a was determined by X-ray structure analysis of a crystalline hydrazone derivative (CCDC 1966948 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre, see the Supporting Information for details).
- 22
- 22aJ. Tsuji, I. Minami, I. Shimitzu, Chem. Lett. 1983, 12, 1325;
- 22bD. C. Behenna, B. M. Stoltz, J. Am. Chem. Soc. 2004, 126, 15044.
- 23For a recent report on a related strategy, see: J. Masson-Makdissi, Y. J. Jang, L. Prieto, M. S. Taylor, M. Lautens, ACS Catal. 2019, 9, 11808.